- Visibility 74 Views
- Downloads 13 Downloads
- DOI 10.18231/j.ijpo.2024.060
-
CrossMark
- Citation
Study of microvascular density in carcinoma of breast
Introduction
The development and progression of a tumour is a complex process involving multiple genetic alterations that confer malignant characteristics. These include not only the tumour's ability to grow and replicate independently but also its interaction with the surrounding tissue, including the promotion of sustained angiogenesis—the formation of new blood vessels from existing ones.[1] Angiogenesis is crucial for supplying nutrients and oxygen to cancer cells and facilitating the removal of waste products, thereby promoting breast cancer’s growth and metastasis.[2]
Breast carcinoma is the most common invasive cancer in females worldwide. In 2020, approximately 2.3 million new cases of female breast cancer were diagnosed globally, with 685,000 deaths attributed to the disease.[3] The incidence is rising in developing regions due to increased life expectancy, urbanization, and adoption of Western lifestyles. It is now the most common cancer in most cities of India. In Manipur, breast cancer constituted 16.4% of all female cancers as per the Population Based Cancer Registry, 2018.
Extensive laboratory research indicates that angiogenesis plays a pivotal role in the development, invasion, and metastasis of breast cancer. Experimental studies have shown that stimulating angiogenesis in tumour cells enhances tumour growth and metastasis while inhibiting angiogenesis suppresses these processes.[4]
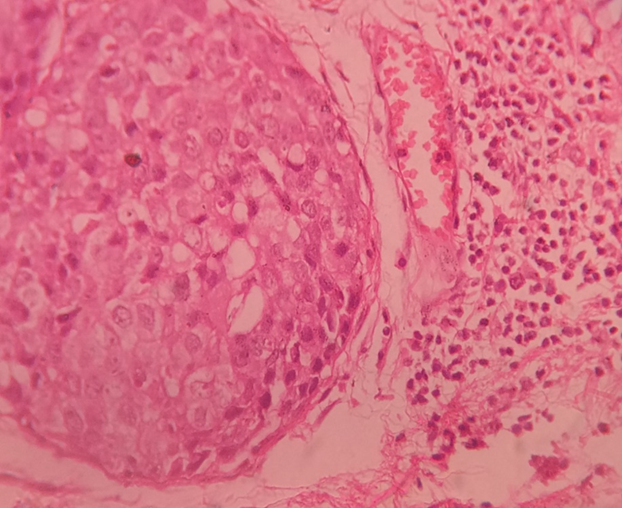
Microvascular density (MVD) assessment is a widely used technique to quantify angiogenesis within breast tumours.[5] The method involves identifying areas of high neovascular density (hot spots) and counting individual microvessels using CD34 immunostaining, which highlights endothelial cells.[6], [7] Studies comparing cancerous tissue with normal tissue reveal elevated MVD in breast cancer, particularly in tumours with metastases to axillary lymph nodes.[8]
The quantity of immunohistochemically highlighted microvessel profiles was subjectively categorised by vascular grading. Standard criteria for identifying a vessel profile were used, which included any stained endothelial cell or cluster of endothelial cells that was distinct from adjacent microvessels. Vessel lumens were not necessary for defining a structure as a microvessel. Microvessels in necrotic or sclerotic tumour areas and immediately adjacent unaffected breast tissue were excluded from vessel evaluations. Three distinct tumour areas with the highest number of discrete microvessels, or hot-spots, were identified. Each hot-spot area was assessed, and the number and area of vessel profiles within the hot-spots influenced the final vascular grade. Thus, areas with high angiogenic activity, characterized by many microvessels, where larger vessel profiles contribute to a higher vascular grade.
Grade 1 (low angiogenesis) was noted when the combined area of the three hotspots contained a small number of endothelial-stained microvessel profiles. This grade is typically assigned to tumors without any significant hot-spots.
Grade 2 (intermediate angiogenesis) was recorded when the combined area of the three hotspots had a moderate number of vessel profiles. This grade is usually given to tumors with one highly vascular hot-spot and two hot-spots with few microvessels.
Grade 3 (high angiogenesis) was registered when the combined area of the three hot-spots contained numerous vessel profiles with large average areas or perimeters.[9]
Intratumoral microvascular density (iMVD) correlates with tumor aggressiveness, including increased metastatic potential and reduced survival rates. Several prognostic factors, such as the number of positive axillary nodes, tumor size, grade, lymphatic and vascular invasion, and hormone receptor status, are traditionally used to predict breast cancer recurrence and survival.[10], [11]
The study aimed to measure microvascular density using CD34 immunohistochemistry in breast carcinomas and investigate its correlation with established prognostic factors.
Aims & Objectives
To measure the microvascular density in carcinoma of breast.
To determine its correlation with the prognostic factors-age, morphological types, tumor size, lymph node metastasis, tumor grade, lymphovascular invasion and ER and PR status.
Materials and Methods
A cross sectional study was conducted among 100 cases of breast cancer for a period of five years from July 2017 to June 2022 in the Department of Pathology, RIMS in coordination with the Department of Surgery, RIMS. All the samples of breast carcinoma tissues which were sent for HPE and IHC from both outpatient and inpatient were included however samples from post-radiotherapy, post-chemotherapy, patients with other co-existing malignancies both present and past and patients with any debilitating diseases like AIDS, TB, etc with a diagnosis of carcinoma in situ were excluded from the study.
Samples were collected as per the guidelines of inclusion and exclusion criteria and informed consents were taken before including the participants in the study.
Counting of micro vessel
Micro vascular density (MVD) was quantified using a light microscope focused on a specific area of invasive tumor measuring 0.74 mm², selected for its representation of the highest density of micro vessels (neovascular hot spot). Prior to counting, endothelial cells lining the micro vessels were identified using CD34 immunostaining, recognized as the most reliable method for highlighting endothelial cells in various laboratory settings.
To determine MVD, the average number of vessels was calculated from four separate fields within the designated area. This mean vessel count represented the final MVD value. The calculation involved dividing the total number of vessels counted (mean microvessel count) by the microscopic field area corresponding to each magnification level (in mm²).[12]
Data management and statistical analysis
Statistical analysis was performed using SPSS Version 21, employing descriptive statistics and Pearson's correlation coefficient to evaluate associations between microvascular density and prognostic factors. p value < 0.05 was considered significant in the study.
Results
During the study period a total of 100 Breast carcinoma specimens were analysed. Histopathological examination and Immunohistochemical analysis of the specimen were done. The baseline characteristics of the Breast carcinoma patients were shown in ([Table 1]).
|
Characteristics |
No of cases(%) |
Mean MVD + SD |
P-value |
|
Age |
|
|
< 0.05 |
|
<50 |
60 (60.00%) |
15.19 + 4.37 |
|
|
>50 |
40 (40.00%) |
21.08 + 5.35 |
|
|
Morphological types |
|
|
>0.05 |
|
Infiltrating Ductal carcinoma |
92(92.00%) |
17.86 + 5.6 |
|
|
Others |
8(08.00%) |
13.93 + 1.9 |
|
|
Tumor size |
|
|
>0.05 |
|
< 3cm |
68(68.00%) |
18.02 + 5.84 |
|
|
>3cm |
32(32.00%) |
16.54 + 4.94 |
|
|
Metastatic axillary Lymph node status |
|
|
< 0.05 |
|
Positive |
12(12.00%) |
23.58 + 5.76 |
|
|
Negative |
88(88.00%) |
16.72 + 5.06 |
|
|
Tumor grade |
|
|
>0.05 |
|
I |
30(30.00%) |
16.58 + 5.22 |
|
|
II |
60(60.00%) |
17.57 + 5.87 |
|
|
III |
10(10.00%) |
20.30 + 4.52 |
|
|
Lymphovascular invasion |
|
|
>0.05 |
|
Positive |
6(06.00%) |
23.00 + 7.51 |
|
|
Negative |
94(94.00%) |
17.27 + 5.37 |
|
|
ER and PR status |
|
|
<0.05 |
|
Positive |
56(56.00%) |
13.29 + 2.00 |
|
|
Negative |
44(44.00%) |
22.96 + 3.49 |
|
|
Her2 status |
|
|
<0.05 |
|
Positive |
62(62.00) |
14.45 ±3.12 |
|
|
Negative |
38(38.00) |
18.36 ± 7.21 |
The age ranged from 30 years to 80 years and the mean age of the study population was 46.66 years. Out of the 100 cases, 60(60.00%) were < 50 years and 40(40.00%) were >50 years which as depicted in ([Table 1]).

|
Age Group |
Number of cases |
Percentage |
|
31-40 |
20 |
20 |
|
41-50 |
43 |
43 |
|
51-60 |
10 |
10 |
|
61-70 |
20 |
20 |
|
71-80 |
7 |
7 |
Breast carcinoma occurred most frequently in the age group 41-50 years composed of 43.00% and which was followed by 20.00% each in the age group of 31-40 years and 61-70 years respectively as depicted in ([Table 2])

Regarding the size of the tumors as shown in [Figure 2], it was more in number with size < 3cm composed of 68.00% followed by tumor size of > 3cm with 32.00%.

Most of the cases in this study as depicted in ([Figure 3]), were of infiltrating ductal carcinoma comprising of 92.00% followed by other cases 08.00%.
|
Grade |
Number of cases |
Percentage |
|
GradeΙ |
30 |
30 |
|
GradeⅡ |
60 |
60 |
|
GradeⅢ |
10 |
10 |
Histological grading was done in all the cases. As shown in [Table 3], 60(60.00%) cases were found to have Grade II disease followed by 30(30.00%) with Grade I. Grade III diseases was seen in 10 cases (10.00%).

Estrogen receptor, Progesterone receptor and Her2-neu receptors status were shown in ([Figure 4]), in which 56(56%) were ER and PR positive cases and 44(44%) were negative cases. Her2-Neu positivity was 62 cases (62%) while negativity was 38 cases (38%). Six cases (06.00%) show lymphovascular invasion by the tumour.([Table 4])
|
|
Number of cases |
Percentage |
|
Positive |
6 |
6 |
|
Negative |
94 |
94 |
|
|
Number of cases |
Percentage |
|
Positive |
88 |
88 |
|
Negative |
12 |
12 |
Twelve cases (12.00%) show metastatic axillary lymph node involved by the tumor. ([Table 5])
|
Prognostic factors |
Tumour size |
Metastatic axillary lymph node status |
Tumour Grade |
Lymphovascular invasion |
ER and PR |
Her2Neu status |
|
Pearsons correlation |
0.12 |
0.40 |
0.17 |
0.11 |
0.87 |
0.32 |
|
p-value |
0.39 |
0.004 |
0.23 |
0.43 |
0.00 |
0.01 |
The [Table 6] shows the correlation of MVD and P value with other prognostic factors. Significant correlation is indicated at p value < 0.05 level. MVD and Metastatic axillary lymph node showed significant relationship with p value of 0.004. The p value was also significant with value of 0.00 between MVD and ER PR receptors. Her2-Neu receptor was also a significant prognostic factor in the present study with p value of <0.05.



Discussion
Angiogenesis has gained attention as a prognostic indicator in various cancers, including melanoma, prostate, and breast cancer. It is commonly assessed by quantifying MVD in tissue sections immunostained for vascular endothelial cell markers like CD34, CD31, or Factor VIII.[11] Studies have suggested that high MVD correlates with increased tumour aggressiveness and poorer prognosis, particularly in early-stage breast carcinoma.[13] Evaluation of MVD using CD34 staining, which has shown superior staining consistency compared to CD31, is crucial in understanding tumour angiogenic potential.
In the present study, CD34 which is the marker for microvessel density stained the micro vessels greater and more intensely as was shown by a study done by L Martin et al[7] in 1997. Their study utilize CD31 and CD34 for the measure of microvessel density. There are some limitation of using CD31. It can react mildly with fibroblasts and some plasma cells and staining failure can reach 20% in routinely fixed breast specimens. CD34 has the same characteristic as CD31 but without high rate of staining failure.
The Chalkley method, a technique for assessing intratumoral vascularization, has demonstrated effectiveness in estimating a tumour's angiogenic potential as evident by studies done by H P Dhakal et al[8] and Fox B et al.[14] Visual scoring methods have also been utilized in the present study as it was found to be a practical and reliable method.
Since the pioneering work by Weidner et al.[6] in 1991, subsequent studies have further explored the prognostic implications of angiogenesis in breast cancer. This study evaluated MVD in relation to various clinicopathological factors such as patient age, tumor size, histological grade, lymph node status, estrogen receptor (ER) status, and vascular invasion. Notably, there was a significant correlation between MVD and patient age, with older patients showing higher MVD, consistent with previous findings.
In this study we found MVD was increasing significantly with age (p value <0.05) which was similar to that found by Maxine Orre et al.[15]
Most cases in this study were of infiltrating ductal carcinoma (92%), and while there was an observable trend of increasing MVD with higher histological grade, statistical significance was not achieved which was in line with findings of Biesaga et al.,[16] and Miliaras et al.[17] while Dhakal et al.[8] observed a significant correlation with mean vascular count and histological grade. Interestingly, significant associations were found between higher MVD and the presence of lymphovascular invasion, indicating a potential role of angiogenesis in facilitating tumour spread through vascular invasion.
Micro vessel density was found to be higher in the presence of lymphovascular invasion but without any association between microvascular density and lymph node metastasis. This result implies that even though tumour angiogenesis might act as prerequisite for vessel invasion, tumour cells may need to acquire additional trait to enable them establish early metastasis in the lymph nodes in line with the invasion-metastasis cascade theory as showed by Hanahan and Weinberg.[1]
When we compared MVD with lymph node involvement we found that there was an increase in mean vascular density of tumour from histological grade I to grade III compared to those with no metastasis with p value of 0.004 similar to studies done by Horak et al.,[18] Weidner et al.,[6] and Bosari et al.[19] who found significant relationship between increased MVD and metastasis to lymph node.
Further analysis revealed that ER-/PR- tumours exhibited significantly higher MVD compared to ER+/PR+ tumours, aligning with prior research linking hormone receptor status to angiogenic activity by Biesaga et al.,[16] Parentes JB et al.[20]
This study highlights the importance of MVD as a prognostic indicator in breast carcinoma, suggesting its potential utility in guiding treatment decisions. However, the study's limitations, including sample size and antibody selection, warrant consideration in interpreting these findings. Future prospective studies with larger cohorts are needed to validate these observations and explore MVD's role further in clinical practice.
Conclusion
This study examined angiogenesis using microvascular density (MVD) in patients with invasive breast cancer, employing antibodies directed against CD34. MVD demonstrated significant correlations with key prognostic factors such as tumor size, metastatic axillary lymph node status, histological grade, lymphovascular invasion, and Estrogen and Progesterone receptor status. These findings suggested that higher microvessel counts are associated with older age (>50 years). Furthermore, the study identified higher MVD in patients with lymph node metastasis compared to those without metastasis. Additionally, tumors negative for Estrogen receptors exhibited significantly greater microvessel counts. Interestingly, tumor neovascularization appeared independent of tumor size, histological grade, lymphovascular invasion, and morphological types. Variations in results regarding the relationship between MVD and prognostic parameters could be attributed to differences in microvessel counting methods and the antibodies used for assessment. Moreover, the quantitative determination of microvessel density holds significance not only for prognostic assessment but also for predicting responses to anti-angiogenic therapies. Standardizing the method of microvessel counting and the selection of antibodies is crucial for accurately assessing MVD and optimizing treatment strategies. This standardization may aid in identifying high-risk patients who could benefit from adjuvant therapy and in evaluating the efficacy of anti-angiogenic drugs in breast cancer treatment.
Source of Funding
None.
Conflict of Interest
None.
Ethical Approval
This study was conducted under Ethics Committee refno.A/206/REB-Comm(SP)/RIMS/2015/209/77/2016.
References
- D Hanahan, RA Weinberg. The hallmarks of cancer. Cell 2000. [Google Scholar]
- G Bergers, LE Benjamin. Tumorigenesis and the angiogenic switch. Nat Rev Cancer 2003. [Google Scholar]
- M Arnold, E Morgan, H Rumgay, A Mafra, D Singh, M Vignat. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022. [Google Scholar]
- J Folkman. Tumor Angiogenesis: Therapeutic implication. N Eng J Med 1971. [Google Scholar]
- A Thielemann, Z Kopczynski, V Filas, J Breborowicz, S Grodecka-Gazdecka, A Baszczuk. The Determination of VEGF and MVD among Patients with Primary Breast Cancer. Pathol Oncol Res 2008. [Google Scholar]
- N Weidner, JP Sample, WR Welch, J Folkman. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med 1991. [Google Scholar]
- L Martin, B Green, C Renshaw, D Lowe, P Rudland, SJ Leinster. Examining the technique of angiogenesis assessment in invasive breast cancer. Br J Cancer 1997. [Google Scholar]
- HP Dhakal, A Bassarova, B Naume, I Borgen, M Synnestvedt, R Kaaresen. Breast carcinoma vascularity: a comparison of manual microvessel count and Chalkley count. Histol Histopathol 2009. [Google Scholar]
- PL Fitzgibbons, DL Page, D Weaver, AD Thor, DC Allred, GM Clark. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement. Arch Pathol Lab Med 1999. [Google Scholar]
- G Agarwal, P Ramakant. Breast Cancer Care in India. The current scenario and the challenges for future. Breast Care 2008. [Google Scholar]
- T Moore, AH Lee. Expression of CD34 and bcl2 in phyllodes tumors, fibroadenomas and spindle cell lesions of the breast. Histopathology 2001. [Google Scholar]
- B Uzzan, P Nicolas, M Cucherat, GY Perret. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res 2004. [Google Scholar]
- S Banerjee, M Dowsett, A Ashworth, LA Martin. Mechanisms of disease: angiogenesis and the management of breast cancer. Nat Clin Pract Oncol 2007. [Google Scholar]
- SB Fox, RD Leek, MP Weekes, RM Whitehouse, KC Gatter, AL Harris. Quantitation and prognosis value of breast cancer angiogenesis: comparison of microvessel density, Chalkley count and computer image analysis. J Pathol 1995. [Google Scholar]
- M Orre, B Susil, PA Rogers. Microvessel density and vascular basement membrane immunostaining in tumors of the breast. Angiogenesis 1999. [Google Scholar]
- B Beisaga, J Niemiec, M Ziobro. Microvessel density and status of p 52 proteuin as potential prognostic factors for adjuvant anthracyclin chemotherapy in retrospective analysis of early breast cancer patient group. Pathol Oncol Res 2012. [Google Scholar]
- D Miliaras, A Kamas, H Kalekou. Angiogensis in invasive breast carcinoma: is it associated with parameters of prognostic significance. Histopathol 1995. [Google Scholar]
- E R Horak, R Leek, N Klenk, S Lejeune, K Smith, N Stuart. Angiogenesis, assessed by platelet/endothelial cell adhesion molecule antibodies, as indicator of node metastases and survival in breast cancer. Lancet 1992. [Google Scholar]
- S Bosari, AK Lee, RA Delellis, BD Wiley, GJ Heatley, ML Silverman. Microvessel quantitation and prognosis in invasive breast carcinoma. Hum Pathol 1992. [Google Scholar]
- Jb Parentes-Vieira, PV Lopes-Costa, CG Pires, AD Santos, J Pereira-Filho, B da Silva. Quantification of angiogenesis in estrogen receptor-positive and negative carcinoma. Int Semin Surg Oncol 2007. [Google Scholar]
