- Visibility 35 Views
- Downloads 5 Downloads
- DOI 10.18231/j.ijpo.2022.073
-
CrossMark
- Citation
Immunohistochemical phenotype of fallopian tubes in patients with different grades of serous ovarian carcinoma
- Author Details:
-
Tamar Dzotsenidze
-
Arsen Gvenetadze
-
Mariam Gachechiladze
-
George Burkadze
-
Shota Kepuladze *
Introduction
Ovarian cancer is seventh most common and number one deadliest gynaecologic malignancy around the world.[1] In Georgia, it is fifth most common cancer, with the incidence of 10.9 per 100 000 inhabitants.[2] Majority of ovarian tumors are epithelial in origin and are classified according to development site.[3] The most common subtype of epithelial ovarian tumors is serous carcinoma, representing about 75% of cases, followed by mucinous carcinoma, representing about 25% of cases. The death cases are mostly attributed to high grade serous ovarian carcinomas, with 20 times more frequency compared to other epithelial tumors.[4] Fallopian tube carcinomas represent more rarity, according to official statistics, the incidence of primary carcinomas of fallopian tube are about 0.36-0.41 per 100 000 inhabitants around the world.[2] Ovary is developed from several embryonic structures, including coeloemic epithelium, subcoelomic mesoderm and primordial germ cells, as well as yolk sac endoderm.[5] Several theories try to explain how ovarian mesothelial cells can undergo through epithelial metaplasia and then dysplasia. However, none of the theories can reliably demonstrate the pathogenesis of high grade serous ovarian tumors.[6] For example, it is known that low grade serous ovarian carcinoma can be developed from benign cystadenoma, however the development of high grade serous carcinoma from low grade serous carcinoma is not confirmed. In addition, many investigators differentiate these two entities as independent lesions, with different origin and molecular profile. During recent years, there is an accumulating evidence that high grade ovarian carcinoma is developed from the fallopian tube epithelial lesions. However, it is not completely understood and still represents the subject of investigation.[7] In support of this theory there is the data available, indicating that in cases of prophylactic salpingo-oophorectomy from patients with BRCA1/2 mutation, incidental fallopian serous lesions are detected.[8] Several studies indicate that these lesions are characterised with similar molecular profile to high grade serous carcinomas, including the loss of p53 or overexpression, known as ’’p53 signature’’.[8] In addition, independent of BRCA1/2 mutation status ’’p53 signature’’ is detected in the fimbriated ends of fallopian tubes, in patients with high grade serous ovarian carcinoma.[9] The detailed immunohistochemical phenotype of matched fallopian tubes and different types of ovarian carcinomas with different malignancy grades has not been yet investigated. The aim of our study was to investigate the expression of proliferation, apoptosis, hormone receptors, epithelial, mesenchymal and stem cell markers in serous and mucinous ovarian carcinomas with different malignancy grade and in matched fallopian tubes.
Materials and Methods
Anonymised formalin fixed and paraffin embedded (FFPE) tissue blocks and haematoxylin and eosin (H&E) stained slides with matched fallopian tubes and ovarian cancer were available in 225 cases, including mucinous borderline tumor (n=25), mucinous carcinoma (n=15), serous borderline tumor (n=90), low grade serous carcinoma (n=72) and high-grade serous carcinoma (n=48). H&E stained slides were revised and evaluated by two independent pathologists (G.B. and M.G.). Fallopian tubes were dissected according to SEE-FIM protocol (Sectioning and Extensively Examining the FIM briated End). Informed consent for the use of FFPE material and associated data have been retrieved from each patient and approved by Ethics Committee of Tbilisi State Medical University (N2-2022/95)
Standard immunohistochemical staining method was used to detect markers of proliferation (Ki67), apoptosis (Bcl2, p53), hormone receptors (ER) and stem cells (CD44). Whole slide images (WSIs) were acquired with Leica Aperio whole slide scanner.
Scanned WSIs were imported in freely available digital pathology software QuPath V0.3.0. After adjustment of chromogenic staining vectors, tissue slides were annotated and total number of tumor cells were detected, followed by the positive cell detection algorithm. Finally, the ratio between total tumor cells and positive tumor cells have been calculated for each marker and the percentage of positive tumor cells have been recorded.
Marker positive cell count <10% has been considered as low and marker positive cells ≥10% has been considered as high. Comparisons between different study groups have been assessed using Mann-Whitney U and Kruskal-Wallis test, non-parametric correlations have been calculated with Spearnan’s rank test. All statistical tests have been performed in SPSS V20.00.
Results
Histopathological investigation of fallopian fimbriae two major types of lesions have been revealed: serous intraepithelial neoplasia and serous carcinoma in situ. Fallopian tube serous intraepithelial neoplasia has been detected in 10/90 (11.1%) of ovarian serous borderline tumor cases, 15/72 (20.8%) of ovarian low grade serous carcinoma cases and in 3/48 (6.25%) of high grade serous carcinoma cases. Fallopian tube serous carcinoma in situ, has been detected in 4/90 (4.4%) of serous borderline tumor cases, 8/72 (11.1%) of low grade serous carcinoma cases and in 15/48 (31.5%) of high grade serous carcinoma cases. In total, fallopian tubes without apparent histopathological changes has been detected in 76/90 (84%) of serous borderline tumors, 49/72 (68%) of low grade serous carcinomas and in 30/48 (62%) of high grade serous carcinomas. Fallopian tube lesions have not been detected in cases of mucinous ovarian tumors ([Figure 1]A). The presence of fallopian tube carcinoma in situ has been significantly correlated with the presence of high grade serous ovarian carcinoma (Spearman’s r=0.44, p<0.001). There was no significant correlation between the presence of fallopian tube lesions and low grade serous carcinoma of the ovary.
Immunohistochemical investigation has been performed in ovarian serous tumors and matched fallopian tubes. Correlation analysis revealed, that the Ki67 proliferation index, p53 mutation status, Bcl2 apoptotic index, ER expression and CD44 stem cell presence in ovarian tumors is significantly associated with matched markers in fallopian tubes. In particular, there was a positive correlation between Ki67 in ovarian tumors and Ki67 in fallopian tubes, including no pathological changes and epithelial lesions (r=.675, p<0.001). Similar results have been found in: p53 Ovary vs. p53 fallopian tubes (r=.70, p<0.001); Bcl2 (r=.597, p<0.001), ER (r=.48, p<0.001) and CD44 (r=.23, p<0.001). Described and additional significant correlations are given in [Table 1].
|
Spearman's Rho |
Ki67 FT |
p53 FT |
CD44 FT |
Bcl2 FT |
ER FT |
|
|
Ki67 OvCa |
Correlation Coefficient |
.675** |
.371** |
.201** |
-.496** |
-.396** |
|
Sig. (2-tailed) |
.000 |
.000 |
.000 |
.000 |
.000 |
|
|
N |
260 |
260 |
210 |
210 |
210 |
|
|
p53 OvCa |
Correlation Coefficient |
.507** |
.709** |
.301** |
-.405** |
-.474** |
|
Sig. (2-tailed) |
.000 |
.000 |
.000 |
.000 |
.000 |
|
|
N |
210 |
210 |
210 |
210 |
210 |
|
|
CD44 OvCa |
Correlation Coefficient |
.522** |
.386** |
.234** |
-.549** |
-.127 |
|
Sig. (2-tailed) |
.000 |
.000 |
.000 |
.000 |
.067 |
|
|
N |
210 |
210 |
210 |
210 |
210 |
|
|
Bcl2 OvCa |
Correlation Coefficient |
-.532** |
-.389** |
-0.290 |
.597** |
.295** |
|
Sig. (2-tailed) |
.000 |
.000 |
.000 |
.000 |
.000 |
|
|
N |
210 |
210 |
210 |
210 |
210 |
|
|
ER OvCa |
Correlation Coefficient |
-.057 |
-.206** |
-0.390 |
.233** |
.503** |
|
Sig. (2-tailed) |
.414 |
.003 |
.000 |
.001 |
.000 |
|
|
N |
210 |
210 |
210 |
210 |
210 |
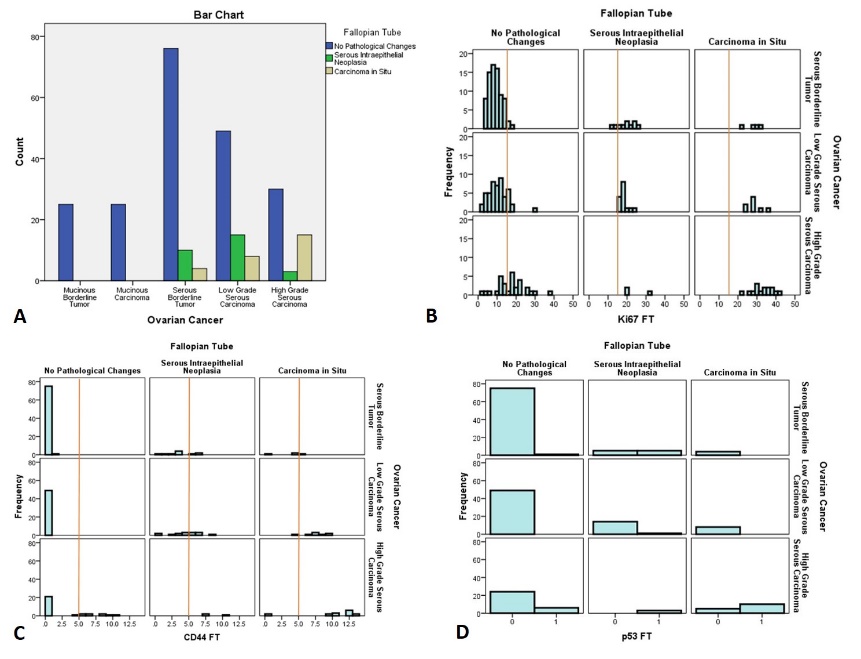
Further immunohistochemical analysis of fallopian tubes without apparent histopathological changes revealed, that immunophenotype of fallopian tubes without apparent histopathological changes varied according to matched ovarian lesion. Particularly, in cases of histologically normal fallopian tubes matched with borderline serous tumors only 3/76 (3.9%) cases showed Ki67 labelling index >15%, in normal fallopian tumors with matched low grade serous carcinoma 9/49 (18.3%) cases showed Ki67 labelling index >15% and in normal fallopian tubes with matched high grade ovarian serous carcinoma 20/30 (66.7%). Detailed distribution of Ki67 labelling index is given in [Figure 1] B. Stem cell marker CD44 expression was varied from 0 to 1% in all fallopian tubes without apparent histopathological changes from patients with borderline serous tumor and low grade serous carcinoma, whilst in patients with high grade serous carcinoma CD44 expression was more conspicuous and varied between 4 to 11% in 6/30 (20%) cases. Detailed distribution of CD44 is given in [Figure 1] C. P53 mutations has not been detected in normal fallopian tubes from patients with borderline ovarian tumors and low grade serous carcinomas, however in patients with high grade serous carcinomas p53 mutations have been detected in 6/30 (20%) of matched normal fallopian tubes. The detailed distribution of p53 is given in [Figure 1] D. Bcl2 apoptotic marker and hormone receptors (ER and PR), did not show any significant relationship between normal fallopian tubes and matched ovarian lesions.
Discussion
We first investigated the distribution of fallopian tube lesions in patients with different types and grades of ovarian carcinoma, including mucinous borderline tumor, mucinous carcinoma, serous borderline tumor, low grade serous carcinoma and high grade serous carcinoma. The results of our study indicated that in case of mucinous borderline tumors and mucinous carcinomas fallopian tubes are not affected, whilst in case of serous ovarian tumors pathological changes can be detected in matched fallopian tubes. Particularly in patients with ovarian serous borderline tumors 11.1% of fallopian tubes show the presence of intraepithelial neoplasia and 4.4% of cases show serous carcinoma in situ, in patients with low grade serous carcinoma of the ovary 20.8% of matched fallopian tubes showed the presence of serous intraepithelial neoplasia and 11.1% showed the presence of carcinoma in situ and in patients with high grade serous ovarian carcinoma 6.25% of fallopian tubes showed the presence of serous intraepithelial neoplasia and 31.5% showed the presence of carcinoma in situ. The results of our study are in line with previous findings, suggesting the different developmental root of mucinous and serous carcinomas of the ovary. In particular, it has been hypothesized that ovarian serous carcinomas are developed from precursor lesions in fallopian tubes.[7], [9] However, with the difference from our study, most of the investigations have been focused on high grade carcinoma of the ovary,[7], [9], [10], [11] whilst we have also investigated the borderline serous carcinomas and low grade serous carcinomas. In addition, we have also examined immunohistochemical phenotype of normal and affected fallopian tubes in patients with borderline, low grade and high grade serous carcinomas. In particular, we have found that there is strong positive correlation between the expression of Ki67, CD44, Bcl2 and ER in matched fallopian tubes and serous ovarian tumors ([Figure 3]), as well as in the presence of p53 mutation. After stratification of fallopian tube immunophenotype as fallopian tubes without apparent histopathological changes and fallopian tube lesions, we have found out that in fimbriated ends of histologically normal fallopian tubes the expression of Ki67 and CD44 is significantly increased in cases of borderline, low grade and high grade serous carcinomas, whilst the presence of p53 mutations as detected by immunohistochemistry have been found in normal fallopian tubes only in cases of matched high grade ovarian carcinomas. Our study indicates that, not only high grade serous carcinomas, but also borderline and low grade serous tumors might be also have their origin in fallopian tubes. However, whether borderline, low grade high grade tumors represent the sequence of events in high grade serous ovarian carcinoma pathogenesis, or these are independent entities remains as open question. Based on the presented data p53 mutation is the breaking point in development of high grade ovarian carcinomas from fallopian tubes. Whilst on the other hand stem cell and proliferation changes are also found in normal fallopian tubes from patients with borderline and low grade serous carcinomas. The limitation of the current study is the non-availability of high throughput genetic testing, which could answer this open question, and we recommend that future investigators also include fallopian tubes from borderline and low grade serous ovarian carcinoma patients in the investigation of fallopian tube origin of ovarian serous carcinoma.
Source of Funding
None.
Conflict of Interest
None.
References
- RL Siegel, KD Miller, HE Fuchs, DVMA Jemal. Cancer Statistics, 2021. Cancer J Clin 2020. [Google Scholar]
- H Sung, J Ferlay, RL Siegel, M Laversanne, I Soerjomataram, A Jemal. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021. [Google Scholar]
- A Chandra, C Pius, M Nabeel, M Nair, JK Vishwanatha, S Ahmad. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med 2019. [Google Scholar]
- P Naik, S Deshmukh, SS Khandeparkar, A Joshi, S Babanagare, J Potdar. Epithelial ovarian tumors: Clinicopathological correlation and immunohistochemical study. J Midlife Health 2015. [Google Scholar]
- B Soygur, DJ Laird. Ovary Development: Insights From a Three-Dimensional Imaging Revolution. Front Cell Dev Biol 2021. [Google Scholar]
- I Nyoman, G Budiana, M Angelina, T Gede, A Pemayun. Ovarian cancer: Pathogenesis and current recommendations for prophylactic surgery. J Turk Ger Gynecol Assoc 2019. [Google Scholar]
- S Kyo, N Ishikawa, K Nakamura, K Nakayama. The fallopian tube as origin of ovarian cancer: Change of diagnostic and preventive strategies. Cancer Med 2020. [Google Scholar]
- SHL George, R Garcia, BM Slomovitz. Ovarian Cancer: The Fallopian Tube as the Site of Origin and Opportunities for Prevention. Front Oncol 2016. [Google Scholar]
- I Shih, Y Wang, T Wang. The Origin of Ovarian Cancer Species and Precancerous Landscape. Am J Pathol 2021. [Google Scholar]
- SI Labidi-Galy, E Papp, D Hallberg, N Niknafs, V Adleff, M Noe. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun 2017. [Google Scholar]
- C Corzo, MD Iniesta, MG Patrono, KH Lu, PT Ramirez. Role of Fallopian Tubes in the Development of Ovarian Cancer. J Minim Invasive Gynecol 2017. [Google Scholar]
