- Visibility 1.4k Views
- Downloads 99 Downloads
- Permissions
- DOI 10.18231/j.ijpo.2023.077
-
CrossMark
- Citation
Insights of hematological parameters in dengue virus-infected patients
Abstract
Background: Early and accurate diagnosis of dengue is critical for prompt treatment and avoiding severe complications. Thus, the present study aimed to evaluate the serological and hematological parameters for predicting the dengue virus infection.
Materials and Methods: A laboratory-based cross-sectional study was conducted among the patients who visited Sumeru Hospital, Lalitpur, Nepal, from July 2022 to June 2023. Blood samples were collected from suspected dengue cases and tested using the rapid diagnostic immunochromatography (ICT) method, and hematological parameters were also assessed. The Mann-Whitney U test compared continuous variables between dengue-infected and non-infected groups. A binary logistic regression analysis was done to evaluate the association of variables for dengue positivity.
Results: When compared to dengue-negative cases, dengue-positive cases had thrombocytopenia, leucopenia, erythrocytosis, high hemoconcentration, low mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC). However, binary logistic regression predicted platelet count, total leucocyte count, MCH, MCHC, neutrophil count and lymphocyte count as significant predictors of dengue positivity.
Conclusion: This study revealed the characteristics and demographics of dengue-positive cases and their associations with hematological parameters. Furthermore, the identified predictive markers can help healthcare professionals diagnose and manage dengue cases more promptly, potentially reducing the disease's burden.
Introduction
Dengue fever, a mosquito-borne viral infection, is a significant public health concern in tropical and subtropical regions, with an estimated 390 million conditions reported each year.[1] Humans are infected by the bites of infected female Aedes mosquitos, primarily Aedes aegypti and Aedes albopictus. Due to its rapid geographic spread via urbanization, international travel, trade, and climate change, along with an increase in the frequency of large-scale outbreaks, dengue fever has become a primary global health concern.[2], [3] Countries with tropical and subtropical climates, such as Africa, the Eastern Mediterranean, South-East Asia, and the Western Pacific, are more vulnerable to dengue infection. The regions most affected include the Americas, South-East Asia, and Western Pacific, with Asia accounting for over 70% of the worldwide disease burden.[4] Previously the World Health Organization (WHO) estimated that over 40% of the world's population is at risk for contracting dengue, and the number of dengue cases has climbed by 30% globally over the previous five decades.[5]
The severity of a dengue infection ranges from minor fever to dengue shock syndrome. Patients with dengue have an acute febrile illness with no localizing symptoms that could be mistaken for other diseases.[6] After the first onset of the fever, up to day nine, the NS1 antigen is present in high concentrations in the blood circulation. While IgM is detectable from Days 3 to 5 of the sickness and lasts 2 to 3 months, IgG appears by Day 14 and persists for life.[7], [8], [9] The recommended method for diagnosing acute dengue infection involves using an in vitro immunochromatographic assay to detect Nonstructural 1 (NS1) protein and IgM and IgG anti-dengue virus antibodies.[10] It has been discovered that the dengue infection changes several hematological indicators. Thrombocytopenia, Leukopenia, raised hematocrit (Hct) and presence of atypical lymphocytes are commonly observed in dengue cases.[11], [12]
Nucleic acid amplification tests are considered as a gold standard test to detect dengue, it is not easily accessible in a resource limited nation like Nepal. Therefore, lateral flow assays (LFA) or immunochromatography (ICT)-based detection methods are commonly used for dengue diagnosis in most developing nations.[13], [14] Even though active dengue detection with ICT is user-friendly, easy to use, and has rapid turnaround results, it has low sensitivity and specificity and increased cross-reactivity that causes more false positives. Hematological parameters can be beneficial as a supportive test for dengue diagnosis in addition to rapid dengue tests via ICT methods.[15] ICT detects dengue-specific antibodies or antigens in a patient's blood, while hematological parameters aids in identifying dengue-related hematological changes such as thrombocytopenia and hemoconcentration.[16], [17]
The conjunction of ICT method and hematological markers can assist healthcare professionals in the early identification and management of dengue fever cases, reducing the risk of severe complications and improving patient outcomes. Furthermore, there are no approved dengue vaccinations or particular antiviral treatments, so patient management must rely on quality supportive care. Accurately identifying dengue infection can help with patient care and community-wide vector control efforts to reduce further transmission.[18] Thus, the objective of the present study was to evaluate the serological and hematological parameters among the patients with dengue virus infection.
Materials and Methods
A laboratory-based cross-sectional study was conducted among the patients who visited Sumeru Hospital, Lalitpur, Nepal, from July 2022 to June 2023. Informed consent was taken from all the study participants.
Inclusion and exclusion criteria
After obtaining informed consent, participants with symptoms of dengue infection and positive dengue in ICT serology were included. Patients who tested negative in ICT serology and having no symptoms of dengue were excluded. However, patients with no signs of dengue and those who tested negative for dengue infection, were taken as a control group.
Specimen collection and processing
Following standard operating procedures, venous blood samples were collected in a K3 EDTA vacuum tube and transported to the laboratory, where blood was mixed gently. A complete blood profile (hemoglobin, RBC and RBC indices, hematocrit, total leukocyte count, differential leukocyte count, and platelets) was performed from an automated hematology analyzer (Sysmex XN-350). Similarly, serum samples were collected in gel clot activator tube to detect dengue infection. Qualitative dengue detection was based on the principle of the rapid immunochromatographic assay (ICT) (Bioline™ DENGUE DUO, Dengue NS1 + IgM/IgG Combo Rapid Test, Abbott). Patients with positive dengue cases were tested for either NS1 or IgM positivity or both NS1 and IgM positivity. Any negative result on any of these profiles was treated as a dengue-negative case. Patients were categorized into positive dengue and negative dengue participants.
Statistical analysis
Data were analyzed using IBM SPSS version 25. Shapiro–Wilk normality test was applied to analyze the data for normal distribution. Continuous variables were presented as median (Q3- Q1). Univariate analysis was performed appropriately using Mann- Whitney U test for the overall analysis between dengue positive and negative groups, and a p-value <0.05 was considered significant. Binary logistic regression was performed as required, and results were represented as crude and adjusted odds ratios with a 95% confidence interval (95% CI). Those variables with p-value <0.05 were considered statistically significant.
Results
Characteristics and demographics of dengue-positive cases
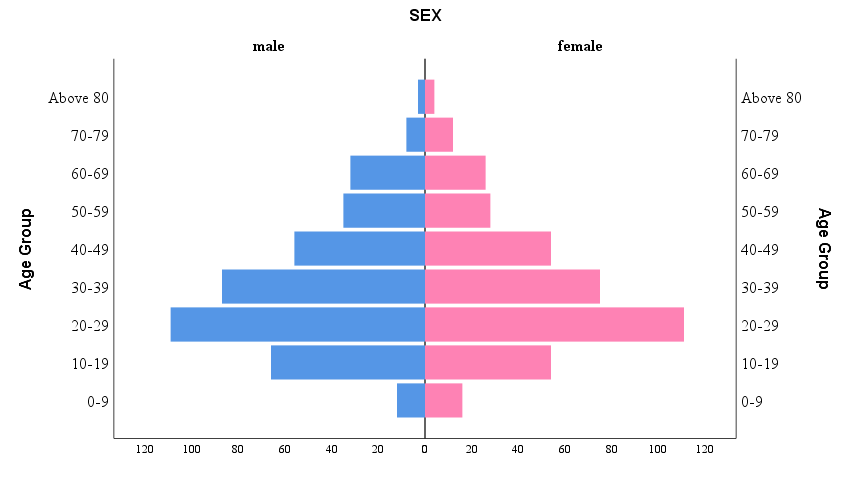
The overall dengue-positive cases were 788. Among them, 73.60% (n=580) were single positive, 22.84% (n=180) were dual positive, and triple positive were found to be 3.56% (n=28) ([Table 1]). The median age of dengue-positive participants was 30 years (Q3- Q1 = 44 years – 22 years). Among 788 dengue-positive subjects, 51.8% (n= 408) were male vs. 48.2% (n= 380) female. Furthermore, the age group 20-29 years was found to have higher positive cases, followed by 30-39 years ([Figure 1]). Mann- Whitney test revealed that the age in the dengue-positive group (median = 30 years) was significantly higher than in the dengue-negative group (median = 28 years); p=0.005 ([Table 2]).
Association of dengue infection with hematological profile
The Mann- Whitney association of hematological profile between the dengue positive and negative groups is presented in [Table 2]. Briefly, in dengue positive group, erythrocytosis, high hematocrit, low MCH, low MCHC, decreased platelet count, decrease in TLC, high neutrophil, low lymphocyte count, low monocyte count, and low Eosinophil were observed than in dengue negative group.
Logistic regression and predictive markers
Binary logistic regression was used to assess the association between laboratory parameters and the outcomes (dengue positive and dengue negative). Independent variables – platelets (p<0.001, OR: 1.000, 95% CI: 1.000-1.000), TLC (p<0.001, OR: 1.000, 95% CI: 1.000-1.000), MCH (p<0.001, OR: 1.163, 95% CI: 1.070-1.263), MCHC (p<0.001, OR: 2.085, 95% CI: 1.751-2.483), Neutrophil (p=0.003, OR: 0.816, 95% CI: 0.713-0.934) and Lymphocyte (p=0.031, OR: 0.861, 95% CI: 0.751-0.986) were added significantly to the model ([Table 3]).
|
Dengue Positive cases |
N |
Total |
|
|
Single positive |
NS1 only |
532 |
580 |
|
IgM only |
28 |
||
|
IgG only |
20 |
||
|
Dual Positive |
NS1+IgM |
162 |
180 |
|
NS1+IgG |
13 |
||
|
IgM+ IgG |
5 |
||
|
Triple positive |
NS1+IgM+IgG |
28 |
28 |
|
Total (Overall Positive) |
788 |
|
Parameters |
Dengue Negative (n= 788) Median (Q3- Q1) |
Dengue Positive (n= 788) Median (Q3- Q1) |
p- value † |
|
Age (years) |
28 (45.0- 19.25) |
30.0 (44.0 -22.0) |
0.005 |
|
Hemoglobin (gm/dl) |
14.1 (15.30-13.32) |
14.2 (15.47- 13.0) |
ns |
|
RBC (X 1012/L) |
4.65 (4.98- 4.37) |
4.93 (5.42- 4.54) |
<0.001 |
|
HCT (%) |
40.60 (43.10- 37.9) |
42.8 (46.6- 38.7) |
<0.001 |
|
MCV (fl) |
86.8 (89.7- 83.7) |
87.40 (91.97- 82.61) |
ns |
|
MCH (pg) |
29.9 (30.8-28.5) |
28.87 (30.15- 27.70) |
<0.001 |
|
MCHC (gm/dl) |
34.6 (35.1- 34.24) |
33.05 (34.36-32.23) |
<0.001 |
|
TLC (cells /cumm) |
6520 (8260- 5590) |
4400 (3500- 5800) |
<0.001 |
|
Neutrophil (%) |
64.1 (69- 58) |
70 (79.75- 57) |
<0.001 |
|
Lymphocyte (%) |
28 (31.53- 24.52) |
24 (37- 15) |
<0.001 |
|
Monocyte (%) |
6.9 (8- 5) |
3 (7- 2) |
<0.001 |
|
Eosinophil (%) |
2 (2.43- 1) |
1 (2- 0) |
<0.001 |
|
Platelets (cells/cumm) |
275000 (336000- 215000) |
170000 (213000-146250) |
<0.001 |
|
Parameter |
Univariate Analysis |
Multivariate Analysis |
||
|
ORC (95% CI) |
p-value |
ORA (95% CI) |
p-value |
|
|
Age |
ns |
ns |
|
|
|
HCT |
0.904 (0.883- 0.925) |
<0.001 |
ns |
ns |
|
MCH |
1.295 (1.222- 1.373) |
<0.001 |
1.163 (1.070- 1.263) |
<0.001 |
|
MCHC |
2.674 (2.388- 2.995) |
<0.001 |
2.085 (1.751- 2.483) |
<0.001 |
|
TLC |
1.001 (1.000- 1.001) |
<0.001 |
1.000 (1.000- 1.000) |
<0.001 |
|
Neutrophil |
0.966 (0.957- 0.975) |
<0.001 |
0.816 (0.713- 0.934) |
0.003 |
|
Lymphocyte |
1.012 (1.002- 1.021) |
0.015 |
0.861 (0.751- 0.986) |
0.031 |
|
Monocyte |
1.328 (1.276- 1.382) |
<0.001 |
ns |
ns |
|
Eosinophil |
1.688 (1.535- 1.857) |
<0.001 |
ns |
ns |
|
Platelets |
1.000 (1.000-1.000) |
<0.001 |
1.000 (1.000- 1.000) |
<0.001 |
Discussion
It is essential to identify the clinical and laboratory factors associated with severe dengue as soon as possible to reduce the likelihood of morbidity and mortality caused by dengue.[19] During the fifth day of an initial infection, dengue-specific antibodies start to manifest, and in most secondary infections, the IgM and IgG-type antibodies cannot be recorded before the third day.[20], [21] However, the NS1 antigen is seen in primary and secondary infections from the first day of dengue fever. Therefore, the NS1 antigen is recognized as a specific viral marker and a reliable parameter for diagnosing dengue.[22] In this study, out of total 788 dengue positive cases, NS1 alone or in combination with either IgM /IgG or with IgM and IgG was positive in 722 (91.6%) of cases. This result is markedly higher than the findings of Kulkarni RD et al. and Joshi A et al.[15], [23] These discrepancies in the dengue infection can be due to the endemicity of dengue continuing to spread towards highland hilly temperate regions of Nepal since the dengue outbreak of 2019.[24] The current study's leading causes of the greater dengue cases are rapid accessibility, quick urbanization, and the Aedes spp—mosquitoes' better adaption to relatively cold environments and the cyclic dengue outbreak with exponentially increased cases.
The current study provides important insights into the characteristics and demographics of dengue-positive cases and their relationship with hematological parameters. The findings show that most dengue-positive cases were single positive, followed by dual-positive patients, and only a small percentage were triple positive. The median age of dengue-positive participants was found to be 30 years, with the age group 20-29 years having the most positive cases, followed by the 30-39 years age group. These findings are consistent with previous studies that found higher dengue incidence rates among young adults, possibly due to increased outdoor activities, mosquito bite exposure, and social behaviors that facilitate disease transmission.[3], [25] Similarly, the study found that males had a slightly higher percentage of dengue-positive cases (51.8%) than females (48.2%). This gender difference in dengue infection has been observed in other studies and could be attributed to differences in male and female behavior, occupation, and exposure to mosquito bites.[26], [27]
This study revealed variations in the hematological parameters in the study population. TLC was significantly lower in dengue-positive patients than in dengue negative. This result is consistent with earlier research that found dengue patients substantially decreased TLC.[28], [29] Similarly, our study observed a significantly lower platelet count in dengue patients concurrently with Rauniyar R et al. and Potts JA et al.[24], [28] Previous studies revealed that increased platelet breakdown and decreased platelet formation during dengue fever are the main factors in thrombocytopenia in dengue infection.[29] However, the higher RBC count, hematocrit, and MCV in patients with dengue were observed in our study, similar to other relevant studies from Pakistan,[30] Ethiopia,[31] and Egypt.[32]
We analyzed routine hematological parameters that may be associated with dengue patients. According to the WHO, the two most significant tests evaluated during dengue infection are hematocrit and thrombocytopenia. Among the analyzed parameters, thrombocytopenia and leucopenia were significantly associated with dengue infection. Thrombocytopenia, which is well correlated with dengue infection as shown by various studies, also remained significant in our study.[33], [34] In a binary logistic regression analysis, RBC indices (MCH, MCHC), neutrophil count and lymphocyte count were independent predictors of dengue positivity. These findings are consistent with previous reports that show dengue infection causes changes in blood cell counts.[26], [35]
The present study has limitations as Polymerase Chain Reaction (PCR) or the enzyme-linked immunosorbent assay (ELISA) for qualitative or quantitative detection could not be used. ICT-based tests are found to be less sensitive than ELISA and RT-PCR, and ICT-based rapid tests have shortcomings, including higher cross-reactivity, which can result in false positive results. Furthermore, the patient's clinical characteristics and disease severity were not assessed. However, the findings from our present study may offer practical routine laboratory indicators for detecting dengue in an endemic location, which may help increase the surveillance of the medical sciences technician performing the dengue diagnosis.
Conclusion
Hematological outcomes like thrombocytopenia, leucopenia, erythrocytosis, high hematocrit, neutrophilia and lymphocytopenia were discovered to be significant predictors of dengue positivity in the study. There is no approved dengue vaccinations or antiviral treatments have been developed so far against dengue infection. Therefore, patient management must rely on prompt diagnosis and quality supportive care. Therefore, the significance of complete hematological parameters in predicting dengue infection can aid in the early diagnosis and management of dengue cases. However, additional research with diverse populations and longitudinal studies are required to validate and extend these findings.
List of Abbreviation
CI: Confidence Interval; DENV: Dengue Virus; Hb: Hemoglobin; IgG: Immunoglobulin G; IgM: Immunoglobulin M; MCH: Mean Cell Hemoglobin; MCHC: Mean Cell Hemoglobin Concentration; MCV: Mean Cell Volume; NS1: Non- Structural Protein 1; ORA: Adjusted Odds Ratio; ORC: Crude Odds Ratio; RBC: Red Blood Cell; WBC: White Blood Cell.
Author's Contribution
Conceptualization was done by TBT and BRB; Methodology was provided by TBT and BRB; Investigation was performed by TBT, SM, SP and MS; Formal analysis was performed by TBT, BRB and Sushant Pokhrel; Original draft was written by TBT and BRB; Review and Editing was performed by TBT, BRB, Sushant Pokhrel; Validation, Supervision and Project Administration was done by TBT.
Data Availability Statement
Data availability statement. The datasets of the current study will be available from the corresponding author upon reasonable request.
Source of Funding
No funding is involved.
Conflict of Interest
The author(s) declare no competing interest.
References
- Bhatt S, Gething P, Brady O, Messina J, Farlow A, Moyes C. The global distribution and burden of dengue. Nature. 2013;496(7446):504-7. [Google Scholar]
- Guzman M, Harris E. Dengue. Lancet. 2015;385(9966):453-65. [Google Scholar]
- Guzman M, Halstead S, Artsob H, Buchy P, Farrar J, Gubler D. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8(12 Suppl):7-16. [Google Scholar]
- . Dengue and severe dengue. . . [Google Scholar]
- Saud B, Adhikari S, Maharjan L, Paudel G, Amatya N, Amatya S. An epidemiological prospective of focal outbreak of dengue infection in Kathmandu, Nepal. J Clin Virol Plus. 2022;2(1). [Google Scholar] [Crossref]
- Chaloemwong J, Tantiworawit A, Rattanathammethee T, Hantrakool S, Chai-Adisaksopha C, Rattarittamrong E. Useful clinical features and hematological parameters for the diagnosis of dengue infection in patients with acute febrile illness: a retrospective study. BMC Hematol. 2018;18(1). [Google Scholar] [Crossref]
- Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbio. 2002;40(2):376-81. [Google Scholar]
- Shu P, Huang J. Current advances in dengue diagnosis. Clin Diagn Lab Immunol. 2004;11(4):642-50. [Google Scholar]
- Gubler D. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11(3):480-96. [Google Scholar]
- Rai D, Azad D, Nautiyal D, Acharya D. Correlation between hematological and serological parameters in dengue patients-an analysis of 2022 cases. Trop J Pathol Microbiol. 2019;5(8):547-54. [Google Scholar]
- Azin F, Gonçalves R, Pitombeira M, Lima D, Branco I. Dengue: profile of hematological and biochemical dynamics. Rev Bras Hematol Hemoter. 2012;34(1):36-41. [Google Scholar]
- Mehta R, Goswami H, Katara R, Patel P, Parikh U, Vegad M. Importance of complete blood count and peripheral smear examination in early diagnosis of dengue patients. J Infect Dis Lett. 2013;2(1). [Google Scholar]
- . Dengue guidelines, for diagnosis, treatment, prevention and control. . 2009. [Google Scholar]
- Yow K, Aik J, Tan E, Ng L, Lai Y. Rapid diagnostic tests for the detection of recent dengue infections: An evaluation of six kits on clinical specimens. PLoS One. 2021;16(4). [Google Scholar]
- Kulkarni R, Patil S, Ajantha G, Upadhya A, Kalabhavi A, Shubhada R. Association of platelet count and serological markers of dengue infection- importance of NS1 antigen. Indian J Med Microbiol. 2011;29(4):359-62. [Google Scholar]
- Blacksell S. Commercial dengue rapid diagnostic tests for point-of-care application: recent evaluations and future needs. J Biomed Biotechnol. 2012;2012. [Google Scholar] [Crossref]
- . Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. . 2009. [Google Scholar]
- Hang V, Nguyet N, Trung D, Tricou V, Yoksan S, Dung N. Diagnostic accuracy of NS1 ELISA and lateral flow rapid tests for dengue sensitivity, specificity and relationship to viraemia and antibody responses. PLoS Negl Trop Dis. 2009;3(1). [Google Scholar] [Crossref]
- Wakimoto M, Camacho L, Gonin ML, Brasil P. Clinical and Laboratory Factors Associated with Severe Dengue: A Case-Control Study of Hospitalized Children. J Trop Pediatr. 2017;64(5):373-81. [Google Scholar]
- Peeling R, Artsob H, Pelegrino J, Buchy P, Cardosa M, Devi S. Evaluation of diagnostic tests: dengue. Nat Rev Microbiol. 2010;8(12 Suppl):30-8. [Google Scholar]
- Schilling S, Ludolfs D, An L, Schmitz H. Laboratory diagnosis of primary and secondary dengue infection. J Clin Virol. 2004;31(3):179-84. [Google Scholar]
- Datta S, Wattal C. Dengue NS1 antigen detection: a useful tool in early diagnosis of dengue virus infection. Indian J Med Microbiol. 2010;28(2):107-10. [Google Scholar]
- Joshi A, Divyashree B, Gayathri B. Hematological parameters in dengue: The serological angle a study. Int J Hematol Res. 2018;4(1):180-4. [Google Scholar]
- Rauniyar R, Prajapati S, Manandhar B, Bastola A, Chalise B, Shrestha S. Dengue virus infection during window period of consecutive outbreaks in Nepal and assessment of clinical parameters. Sci Rep. 2023;13(1). [Google Scholar]
- Huy R, Wichmann O, Beatty M, Ngan C, Duong S, Margolis H. Cost of dengue and other febrile illnesses to households in rural Cambodia: a prospective community-based case-control study. BMC Public Health. 2009;9(1). [Google Scholar]
- Bhattarai B, Mishra A, Aryal S, Chhusyabaga M, Bhujel R. Association of Hematological and Biochemical Parameters with Serological Markers of Acute Dengue Infection during the 2022 Dengue Outbreak in Nepal. J Trop Med. 2023;2023. [Google Scholar] [Crossref]
- Wilder-Smith A, Foo W, Earnest A, Sremulanathan S, Paton N. Seroepidemiology of dengue in the adult population of Singapore. Trop Med Int Health. 2004;9(2):305-8. [Google Scholar]
- Potts J, Rothman A. Clinical and laboratory features that distinguish dengue from other febrile illnesses in endemic populations. Trop Med Int Health. 2008;13(11):1328-40. [Google Scholar]
- Kalayanarooj S, Vaughn D, Nimmannitya S, Green S, Suntayakorn S, Kunentrasai N. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176(2):313-21. [Google Scholar]
- Butt N, Abbassi A, Munir S, Ahmad S, Sheikh Q. Haematological and biochemical indicators for the early diagnosis of dengue viral infection. J Coll Physicians Surg Pak. 2008;18(5):282-5. [Google Scholar]
- Ferede G, Tiruneh M, Abate E, Wondimeneh Y, Gadisa E, Howe R. A study of clinical, hematological, and biochemical profiles of patients with dengue viral infections in Northwest Ethiopia: implications for patient management. BMC Infect Dis. 2018;18(1). [Google Scholar]
- Aziz B, Hassanien S, Abdou A. Clinical and Hematological Effects of Dengue Viruses Infection. Am J Infect Dis Microbiol. 2016;4(4):74-8. [Google Scholar]
- Khan M, Anwar E, Agha A, Hassanien N, Ullah E, Syed I. Factors predicting severe dengue in patients with dengue Fever. Mediterr J Hematol Infect Dis. 2013;5(1). [Google Scholar] [Crossref]
- Chao C, Wu W, Lai Y, Tsai P, Perng G, Lin Y. Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage. PLoS Pathog. 2019;15(4). [Google Scholar] [Crossref]
- Dumre S, Bhandari R, Shakya G, Shrestha S, Cherif M, Ghimire P. Dengue Virus Serotypes 1 and 2 Responsible for Major Dengue Outbreaks in Nepal: Clinical, Laboratory, and Epidemiological Features. Am J Trop Med Hyg. 2017;97(4):1062-9. [Google Scholar]
- Abstract
- Introduction
- Materials and Methods
- Results
- Characteristics and demographics of dengue-positive cases
- Association of dengue infection with hematological profile
- Logistic regression and predictive markers
- Discussion
- Conclusion
- List of Abbreviation
- Author's Contribution
- Data Availability Statement
- Source of Funding
- Conflict of Interest
- References
How to Cite This Article
Vancouver
Thapa TB, Bhattarai BR, Maharjan S, Pokhrel S, Sapkota M. Insights of hematological parameters in dengue virus-infected patients [Internet]. Indian J Pathol Oncol. 2023 [cited 2025 Oct 25];10(4):340-345. Available from: https://doi.org/10.18231/j.ijpo.2023.077
APA
Thapa, T. B., Bhattarai, B. R., Maharjan, S., Pokhrel, S., Sapkota, M. (2023). Insights of hematological parameters in dengue virus-infected patients. Indian J Pathol Oncol, 10(4), 340-345. https://doi.org/10.18231/j.ijpo.2023.077
MLA
Thapa, Tika Bahadur, Bhattarai, Bibek Raj, Maharjan, Sujina, Pokhrel, Sushant, Sapkota, Manisha. "Insights of hematological parameters in dengue virus-infected patients." Indian J Pathol Oncol, vol. 10, no. 4, 2023, pp. 340-345. https://doi.org/10.18231/j.ijpo.2023.077
Chicago
Thapa, T. B., Bhattarai, B. R., Maharjan, S., Pokhrel, S., Sapkota, M.. "Insights of hematological parameters in dengue virus-infected patients." Indian J Pathol Oncol 10, no. 4 (2023): 340-345. https://doi.org/10.18231/j.ijpo.2023.077
