Introduction
Renal cell carcinoma (RCC) is the commonest renal malignancy accounting for 3% of all adult malignancies 1. Renal cell carcinoma (RCC) is the ninth and fourteenth common cancer in men and women, respectively and the sixteenth cause of mortality from cancer worldwide.1 The 5-year prevalence of RCC was 4.44% in Sri Lanka for all ages in 2020.2
The 5th series World Health Organization of adult renal (epithelial) tumour classification includes many subtypes, some incorporating molecular genetic alterations as diagnostic criteria (Table 1).3 The clear cell RCC is the commonest subtype with an incidence of 60-75%.3 The reason for the clear cytoplasm is its lipid and glycogen content which dissolves with routine histological processing.3 High grade tumours and cells adjacent to an area of necrosis or haemorrhage may show eosinophilic cytoplasm3 and some authors have designated them as the eosinophilic variant of ccRCC and as granular RCC in past.4
Table 1
The 5th series World Health Organization renal tumour classification
Since 1970, researchers have come up with various grading systems focusing on the morphology of the nucleus. In 1982 Fuhrman et al. introduced internationally and most widely accepted Fuhrman grading system for nuclear grading of RCC (Table 2).5
Table 2
Fuhrman grading system for nuclear grading of RCC5
It considers three main features; nuclear size (area, major axis, perimeter), nuclear shape (shape factor, nuclear compactness) and nucleolar prominence.5 This is a four-tiered system.5
Fuhrman grade 1 is considered the least aggressive and type 4 is the most aggressive type. Therefore, grades 1–2 are considered as low grade and 3-4 as high grade.5 The Fuhrman grade is considered to have a very low prognostic significance for ChRCC but is highly effective in predicting the biological aggressiveness and metastatic risk of ccRCC and pRCC.5
In 2012, a novel grading system was introduced by the International Society of Urological Pathology (ISUP) for clear cell and papillary RCC at the consensus conference held in Vancouver, Canada.6 It is also a four tiered grading system.6 (Table 3).
Table 3
WHO/ISUP grading system for nuclear grading of RCC 6
In this grading system grade 1 to grade 3 tumours depend on increasing prominence of nucleoli.6
The tumours with extreme nuclear pleomorphism and/or tumour giant cells with or without nucleolar prominence are determined by detection at specific magnifications using a light microscope.7 One high-power field reflecting the greatest degree of nuclear pleomorphism is used for the purpose of grading.3 The rationale for the grading was based on the evidence that ribosome biogenesis determines nucleolar size.8 Nucleolar hypertrophy correlated with the patient outcome.4
Currently, only the ccRCC and pRCC are the two subtypes of RCCs for which WHO/ISUP nuclear grading is validated to be applied. 3 The state of understanding of applying WHO/ISUP grading system is variable in different types of RCC according to the published literature and is summarised as follows in the latest WHO blue book. (Table 4)
Table 4
The state of understanding of applying the WHO/ISUP system for grading in the context of published literature3
Materials and Methods
The objective of this was to describe the types of adult renal cell carcinomas presenting to a tertiary care hospital in Sri Lanka and to assess the intraobserver variability of two nuclear grading systems for clear cell renal cell carcinoma.
The study was a descriptive cross sectional study with an analytical component carried out in the Department of Histopathology of National Hospital of Sri Lanka (NHSL) and the Faculty of Medicine, University of Colombo (FMC); both centres receive a significant number of renal cell carcinoma specimens and referrals from around the country. RCC cases from 1st January, 2016 to 31st December, 2020 were included for the study, from both centres (n = 228).
The archived records of both centres were looked into and all the cases diagnosed as renal cell carcinoma were selected. All the slides representing tumour sections of each case were examined under the light microscope and the most informative slide was chosen. The original histological tumour type and the nuclear grade given by the respective consultant histopathologist at the time of reporting were not recorded or looked into while examining the slides.
Each tumour slide was assessed initially for the tumour typing, based on the described histological features for histologically identifiable tumour types. The cases that appear to require further molecular testing for typing were separated out. This information was entered in a data entry sheet. The data was presented as percentages in relation to the total number of cases.
Afterwards, all the cases diagnosed as clear cell renal cell carcinoma based on histological features were selected to assess the nuclear grading (n = 189) and the selected slide of each case was evaluated under the light microscope. At first the nuclear grading was performed according to the Fuhrman grading system (F – Round 1) and the values were entered in to a data entry sheet. The assessment by the WHO/ISUP grading system was performed after a washout period of two weeks (W – Round 1) to prevent any bias in the assessment. The investigator was blind to the results of the Fuhrman grading while performing the WHO/ISUP grading. The results were recorded separately in to another new data entry sheet.
After another washout period of two weeks the same process was repeated. The sequence of the slide set was altered by shuffling slides to prevent any bias. The second round of nuclear grade assessment by WHO/ISUP system (W – Round 2) was performed after a washout period of two weeks, upon completion of the second round of nuclear grade assessment by Fuhrman system (F – Round 2). Thereby the washout period between the assessments of nuclear grading using a similar system was stretched to a gap of one month in order to prevent bias. The findings of the two rounds were entered in two fresh data entry sheets. The investigator was blind to the results of the two previous rounds.
After performing all four rounds of nuclear grading using both systems, all the data was entered into the SPSS version 25.0 and the Cohen’s kappa of coefficient9 for each grading system was calculated. The values were then compared with the given reference ranges and the level of agreement (hence the intra-observer variability) between the two systems was interpreted.
The ethical clearance for the study was obtained from both ethical review committees of National Hospital of Sri Lanka and Faculty of Medicine, Colombo. Only the H and E stained slides were used for the study and demographic information or any patient identifiers were not disclosed. The wax blocks were also not utilized. Only one slide from each case was retained throughout the study and all the slides were returned to the archives upon completion. Information in the data entry sheets were saved in a password secured software and each case was given a specific reference number different to that of the original laboratory reference number to maintain the confidentiality of the data.
Results
Based on the histological features of the H and E stained representative section of the tumour the diagnosis was made according to the WHO 5th series of classification of adult renal cell carcinomas and their distribution is illustrated in (Figure 1).
Figure 1
The distribution of histological types of RCC observed in the study population
*This group comprises the likely molecularly defined cases based on histology (n = 7)

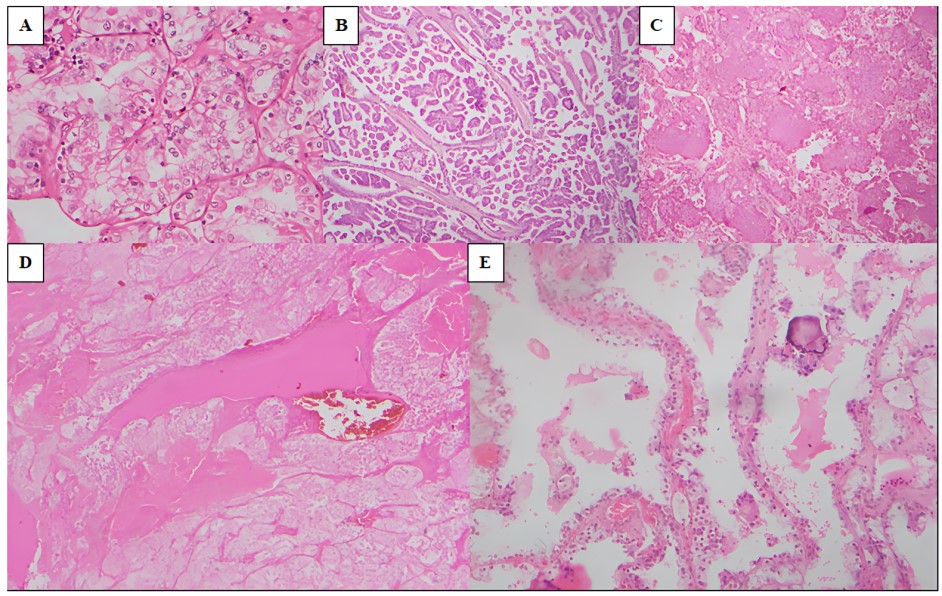
The identified histological subtypes of our study population are as follows (Figure 2).
Clear cell RCC (ccRCC) (82.9%) (Figure 2 A)
Majority of the tumours (189 out of 228) comprised ccRCC. These tumours showed a spectrum of growth patterns including nested, tubular and alveolar architecture. The constituent cells showed abundant clear cytoplasm with variable nuclear features. The background showed a rich and a complex vasculature with large areas of haemorrhage and necrosis in some tumours. Occasionally, foci of sarcomatoid and rhabdoid differentiation were noted.
Papillary RCC (pRCC) (10.5%) (Figure 2 B)
This was the second most common subtype (24 out of 228). Most of these tumours showed papillary structures with fibrovascular cores lined by cuboidal cells. Some tumours showed many foamy histiocytes and psammoma bodies. A few showed a solid growth pattern whilst some showed a biphasic pattern. Brisk inflammation was also seen in a few.
Chromophobe RCC (ChRCC) (2.6%) (Figure 2 C)
Approximately 6 out of 228 tumours belonged to this subtype. The tumour cells were arranged in solid sheets and nests. Most cells were pale with clear cytoplasm. The cell membrane is prominent and showed a plant cell-like morphology. The nuclei were hyperchromatic. Some cells were densely eosinophilic with wrinkled, raisinoid nuclei.
Eosinophilic solid and cystic RCC (ESC-RCC) (Figure 2 D)
Another case showed features of ESC-RCC with solid and cystic areas. The cells were polygonal with voluminous eosinophilic cytoplasm however, cytoplasmic stippling was not prominent. The nuclei are round-oval with mild pleomorphism. Foamy histiocytes, lymphocytes and psammoma bodies were present, but sparse.
Multilocular cystic renal neoplasm of low malignant potential (MCNLMP) (Figure 2 E)
There was a single case composed of variably sized cysts lined by cells with clear cytoplasm. The nuclei were of WHO/ISUP grade 1. There was no evidence of necrosis, mitoses, lymphovascular invasion or sarcomatoid/rhabdoid differentiation.
Figure 2
The morphological spectrum of RCC types observed in the study population; A): ccRCC (H and E x100); B): pRCC (H and E x40); C): ChRCC (H and E x40); D): ESC-RCC (H and E x40); E): MCNLMP (H and E x100)
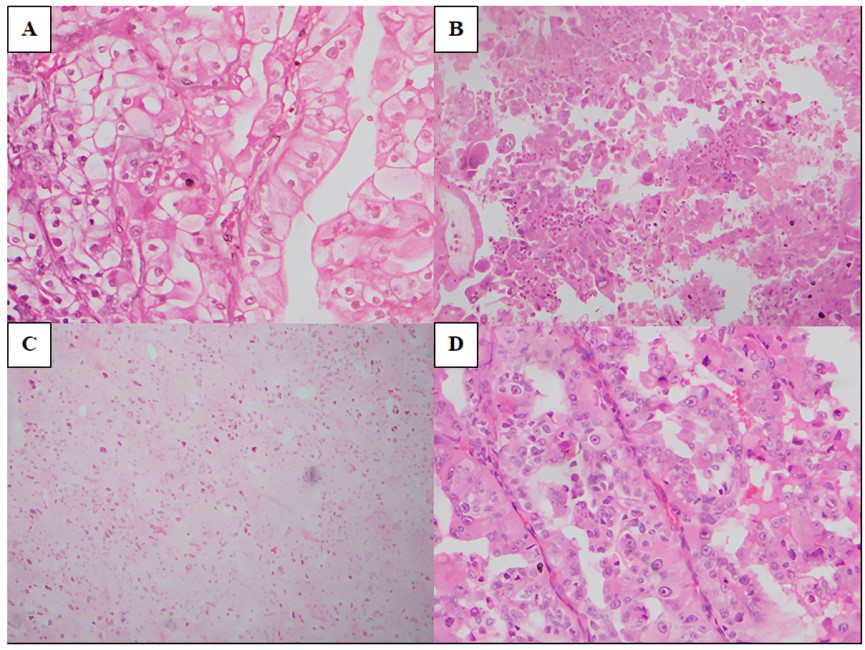
Based on the light microscopic features 7 cases appeared to require further molecular testing for the histological typing. The likely molecularly defined entities identified in the study sample are summarised in (Table 5).
Table 5
The likely molecularly defined entities identified in the study sample
TFE3 rearranged RCC (Figure 3 A)
Two tumours showed the papillary architecture with epithelioid clear cells lining the papillae. Scattered psammoma bodies were noted. The nuclei were vesicular with prominent nucleoli visible at x100 magnification. One showed a mixture of solid and papillary growth patterns.
TFEB rearranged RCC (Figure 3 B)
These tumours showed biphasic morphology. There was an admixture of larger epithelioid cells and smaller cells. The larger cells were at the periphery and arranged in nests while the smaller cells were in the centre clustered around basement membrane material. Entrapment of native renal tubules at their periphery was noted.
SDH deficient RCC (Figure 3 C)
This tumour demonstrated a sheet-like growth comprising compact nests of cells with pale, eosinophilic cytoplasm. The cell borders were not prominent. No microcysts were seen. Entrapment of native renal tubules was seen at the periphery. Some cells showed pale eosinophilic cytoplasmic material. Thee nuclei were of WHO/ISUP grade 4 showing moderate pleomorphism and scattered multinucleated forms.
FH deficient RCC (Figure 3 D)
Both tumours demonstrated papillary morphology. No foamy macrophages were seen. The cells lining the papillae showed abundant, deeply eosinophilic cytoplasm and round, vesicular nuclei with eosinophilic macronucleoli.
Figure 3
The histology of likely molecularly defined entities identified in the study sample; A): TFE3 rearranged RCC (H and E x400); B): TFEB rearranged RCC (H and E x200); C): SDH deficient RCC (H and E x200); D): FH deficient RCC (H and E x400)
During the second phase of the study, nuclear grading was performed on the 189 ccRCC cases using the Fuhrman system and the WHO/ISUP system as described above. The results of the two separate rounds of each system are illustrated (Figure 4).
Majority of the ccRCC were of nuclear grade 2 followed by grade 1 and grade 3. The highest discrepancy in the nuclear grade was also reported among the grade 2 tumours. A total of 18 cases of nuclear grade 4 were reported in all four rounds.
The above data was assessed using SPSS version 25.0 to calculate the level of agreement using Cohen’s kappa of coefficient for each grading system and the results are summarised in (Table 6).
Table 6
Cohen’s kappa (κ) of coefficient of the two grading systems (SPSS version 25.0)
|
Grading system |
Value of Kappa (κ) |
Level of agreement |
95% Confidence Interval |
|
Fuhrman system |
0.856 |
Strong |
0.794 – 0.918 |
|
WHO/ISUP system |
0.953 |
Almost perfect |
0.916 – 0.990 |
According the calculated data the WHO/ISUP system showed the least intra-observer variability in the assessment of nuclear grading in ccRCC with an almost perfect level of agreement (κ = 0.953). The Fuhrman system also showed a strong level of agreement (κ = 0.856).
The number of cases showing a discrepancy in the nuclear grade in each system are summarised in (Table 7).
Discussion
In the recent past, precision medicine and targeted therapies are adopted increasingly in clinical practice, which complements the role of histopathology in prognostication and cancer prediction.3 The morphology still remains the foundation of renal tumour classification which has been attempted in the first part of our study. Depending on the morphology the RCCs have been subtyped; the clear cell comprised the largest group amounting to approximately 83%. Papillary RCC was the 2nd most common subtype (10.5%) followed by ChRCC (2.6%). These figures were concordant with the findings of the study done by Kuthi et al. in 2017 in Hungary, which showed a similar distribution of different RCC subtypes.10 The application of molecular profiling has also made a great impact on renal tumour taxonomy and classification.3 Thus, the tumours which showed histological features suggestive of molecularly defined renal tumour entities were grouped separately; although genetic studies were not carried out to confirm the diagnosis due to lack of facilities for same in our setting. However, this underscores the importance of the availability of appropriate ancillary technology in our pathology laboratories. As it may be challenging in low-income countries like Sri Lanka, the importance of careful selection of cases for further testing by meticulous morphological assessment was highlighted.
Before the introduction of WHO/ISUP system, the Fuhrman system was widely used for the nuclear grading of conventional RCC.6, 11, 12, 13, 14 This 4-tiered system is based primarily on nuclear size, shape and nucleoli.6, 11, 12 The findings would predict the 5-year disease-specific survival probabilities stratified by grade; 50–97% for patients with G1 tumors, 30–90% for patients with G2 tumors, 10–78% for patients with G3 tumors, and 9–66% for patients with G4 tumors.13 This data was supported by other similar studies.11, 14 However, the prognostic stratification has been demonstrated only when patients were clustered into two or three different categories; the major drawback of this system.13
According to the most available studies, the interobserver agreement for Fuhrman system was low to moderate which improved only when it is modified to a three- or two-tiered system.13, 14, 15, 16 The intraobserver agreement was however moderate.13 Furthermore, there were criticisms related to its application, validity, and reproducibility and was largely discouraged to be used in routine practice.17, 18 Interestingly, the findings of our study demonstrated a strong level of intraobserver agreement for Fuhrman system (κ = 0.856 with a 95% confidence interval of 0.794 – 0.918); albeit not as high as the WHO/ISUP system.
The level of intraobserver agreement for WHO/ISUP system on the other hand was almost perfect (κ = 0.953 with a 95% confidence interval of 0.916 – 0.99). This system which was introduced in 2012 has proved to have better reproducibility and a better predictor of prognosis and outcome of patients with RCC.17, 18 According to the latest 5th series WHO blue book, there are different states of understanding of the application of WHO/ISUP grade in RCC subtypes in the context of published literature and the process of validation is still in progress.3, 19 To date, only clear cell and papillary subtypes show validated data for the clinical applicability of WHO/ISUP grading system (category 1).3, 19 Studies have shown that it is not applicable for ChRCC (category 2).3, 19 It is recommended to assign and report WHO/ISUP grading in RCC subtypes where it may be potentially useful (category 3).3 The grading is less relevant in certain aggressive tumours such as collecting duct carcinoma (category 4), and in tumours where low-grade nuclear features are essential for the accurate histological classification (category 6).3 For some tumours, grading may cause confusion and be misleading (category 5) and the significance of grading may be unknown (category 7) 3 It is stated that these tumours might not be graded, or, if graded to add a comment about the lack of its known clinical significance.3
In contrast to WHO/ISUP system, the Fuhrman system requires the assessment of subtle features such as nuclear diameter, nuclear shape, in addition to the nucleolar prominence when assigning the nuclear grade. When multiple features are associated the chance of making errors is more. On the other hand, certain morphometric features such as tumour giant cells, extremely pleomorphic cells and sarcomatoid/rhabdoid differentiation were easily recognizable, and hence assigning the nuclear grade 4 is more straightforward compared to the other 3 grades; as evidenced by the 100% reproducibility observed for both grading systems in both rounds for grade 4 tumours.
The human factors affecting grade interpretation partly be due to poor slide quality and the presence of artefacts. Some of the older slides showed fading of the stains which made the assessment more difficult in the study. Artefacts such as presence of formalin pigment, cracking of mounting agent and entrapment of air bubbles also hindered the assessment in a few slides by obscuring the nuclear details, especially among grade 2 and grade 3 tumours.
In view of the implication of nuclear grade on patient outcome it is crucial to avoid any possible errors in interpretation of grading. As per our findings the errors are much less in WHO/ISUP system compared to Fuhrman system. It could be; though both systems have 4 tiers, features considered for grading is less in WHO-ISUP than in Fuhrman system i.e. presence of nucleoli at different magnifications based on size mainly and nuclear pleomorphism in the former versus nuclear size, shape and nucleoli visible at different magnifications and nuclear pleomorphism in the latter. In addition, the WHO/ISUP system has the advantage of being easier to perform by the busy pathologist as fewer criteria are used and therefore the possibility of inter and intra observer variability is less theoretically even though both showed good intraobserver variability in this study.
The errors related to such complexity and human factors could be prevented by the introduction of an automated system using an artificial intelligence (AI) tool to perform the nuclear grade. Another advantage of the WHO/ISUP is that it is easier to develop an AI tool when only 1-2 criteria are used than when 3 or more criteria are used. The applicability of such a tool however may require validation through a large scale study which may be the future scope.
Conclusions
Majority of the adult RCC in Sri Lanka are of clear cell subtype, followed by papillary and a few chromophobe RCCs. Other subtypes are very rare. Some renal tumours may however, require molecular testing for the confirmation of the diagnosis, hence the necessity to develop facilities for molecular testing in our setting is emphasized.
WHO/ISUP grading has an almost perfect level of intraobserver agreement in assessing the nuclear grade of RCC, through validation in this study. Although discordant with many studies, the Fuhrman system also demonstrated a strong level of intraobserver agreement.
Our study provides information on the distribution of different histological subtypes of adult RCC in Sri Lankan population, based on the data of two main centres of the country over a period of five (05) years. So far no similar study has been performed on a larger scale in Sri Lanka.
This study also proves the fact that the reproducibility of WHO/ISUP system is better compared to the Fuhrman system in the assessment of the nuclear grade; in concordance with the available overseas studies.
Importance of the Study
Only one study, which describes the histological subtypes is available; with regard to the prevalence of RCC types in the Sri Lankan setting as well as of RCC’s that require molecular testing for typing. The knowledge on different histological types as well as those that require molecular testing for typing is valuable for epidemiological purposes as well as for the development of a comprehensive pathology service with facilities for molecular genetic studies capable of diagnosing all types of RCC’s in the country.
Tumour grade is considered as one of the most powerful prognostic factors for ccRCC and pRCC.5 There is no documented study comparing the intra-observer variability with in the two above mentioned grading systems in Sri Lanka, and therefore their utility in our pathology practice. Therefore the assessment of a nuclear grading system which has a higher reproducibility is important for the management and prognostication of RCC in our setting.
Sseveral studies with regard to the nuclear grading of renal cell carcinoma have been done overseas, whereas no similar documentations are found related to the Sri Lankan population in our setting.
Limitations of the Study
As this study was performed retrospectively, it was assumed that adequate sampling has been performed and the slides were representative of the tumour as examination of the entire tumour is necessary for the assessment of the worst nuclear grade and for the histological subtyping of the tumour.
Molecular genetic studies were not performed to confirm the histological diagnosis of the seven (07) tumours which were identified as requiring molecular techniques; hence at least a few of them might be negative for such testing and be re-classified as a pRCC or a ccRCC depending on the morphology.
Tumours which require genetic mutational analysis were excluded from nuclear grading component of the study.
Not being a population based study.
Not having information on the outcome of the patients.
Recommendations
The facilities to perform molecular genetic studies should be established to confirm the diagnosis of cases which require molecular testing. The candidate cases should be carefully selected based on histomorphology to minimise the cost. Extensive sampling and peer-reviewing may be necessary.
The development of artificial intelligence (AI) tools to perform nuclear grade may further improve the reproducibility and potentially facilitate greater consistency in grading by alleviating errors related to human factors.’

