Introduction
Breast masses are uncommon in childhood.1 The majority of them are related to inflammation (infection or abscess) or benign tumours as fibroadenoma.2 Juvenile fibroadenoma is a rare clinical entity, encountered in children and adolescent girls and forms 4% of the total fibroadenomas, and giant juvenile fibroadenoma constitutes only 0.5% of all fibroadenomas.3
The Stanford School of Medicine clearly defined both the entities of juvenile and giant fibroadenoma.4 Juvenile fibro adenoma is circumscribed, often large, breast mass usually occurring in adolescent females with stromal and epithelial hyper cellularity. Giant fibroadenoma is defined as a tumour >500 gms or more than 5cm or replacing at least 80% of the breast.4 Our case satisfies both these definitions.
Somatic mutation in exon 2 of the MED12 gene is identified in 60% of breast fibroadenomas.5, 6
We hereby present a rare case of giant juvenile fibro adenoma in a young pre-pubertal girl aged 10 years. She came with a second breast lump measuring 8x6 cms in the contralateral breast on follow up after eighteen months.
Case Report
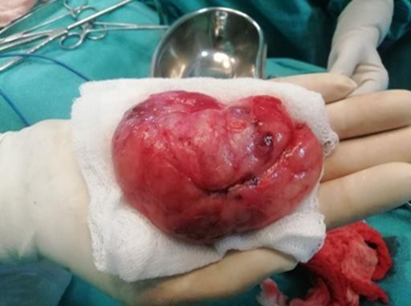
A pre pubertal girl aged 10 years presented with a rapidly growing lump in the left breast since two months and a second lump in right breast 18 months later. Lump in left breast was 10x8 cm in size occupying all the quadrants of breast and right breast lump measured 8x6cms. Lumps were painless, freely mobile firm mass with bosselated surface (Figure 1). No associated nipple discharge or any regional lymphadenopathy was noted. The skin over the breast was tense, shiny with engorged superficial veins.
Ultrasonography of the breast revealed a well-defined, hypo to mixed echogenic mass lesion occupying the entire breast. FNAC yielded highly cellular smears containing duct epithelial cells arranged in tightly cohesive branching sheets. The sheets are lined by myo epithelial cells with presence of numerous bare oval nuclei in the background (Figure 2). It was diagnosed as benign proliferative lesion without atypia suggestive of fibroadenoma.
Figure 2
a): (left lump) b: (right lump): Cellular smears with cohesive branching sheets and bare oval nuclei in background

Resected left breast mass was processed for HPE (Figure 3a) and FFPE tissue block was also subjected to next generation sequencing to identify the causative driver mutation. The patient came with a large lump in the right breast 18 months later, which was cytologically diagnosed as fibroadenoma, which was excised.
Gross Examination
A single grey white to grey brown irregular soft tissue mass (left breast lump 9x7.5x4.5cm and right breast lump 8x6x4cms) with bosselated external surface. Cut section revealed greyish white homogenous areas with irregular septations and cleft like spaces (Figure 3b).
Figure 4
a): Gross appearance of right lumpectomy specimen, b): (in set) Cut section showing grey white areas and cleft like spaces

Microscopic examination
H&E Stained sections show a well circumscribed tumour composed of hyperplastic duct and stromal components. Ducts are elongated and lined by cuboidal epithelium with areas of hyperplasia and apocrine change. Stroma shows increased cellularity, composed of fibroblasts with elongated vesicular nuclei and prominent nucleoli. Extra cellular tissue composed of fibrous and collagen bundles was seen. No mitotic figures and necrosis were appreciated. The final histopathological diagnosis of Giant juvenile cellular fibro adenoma was given. (Figure 5 a, b and c).
Figure 5
a): (Left lump): Ductal and Stromal hyperplasia, b): (left lump) Ducts lined by cuboidal epithelium and focal apocrine change, c): (right lump) stromal hyperplasia

MED 12 exon 2 molecular report
Tissue block was subjected to molecular analysis by PCR – sequencing and c122_139del18/pv41_n46del was detected (Figure 6)
At the time of submission of this report, the patient presented with third swelling in the right breast. FNAC and tru-cut biopsy revealed similar features as previous swellings. Surgery done recently on the third swelling shows another cellular fibroadenoma.
Discussion
According to Stanford School of Medicine, juvenile fibroadenoma of the breast is defined as “circumscribed, often large, breast mass occurring in adolescent females with stromal and epithelial hyper cellularity but lacking the leaf like growth pattern of phyllodes tumour’s”.4
Diagnostic criteria for juvenile fibroadenoma are
Circumscribed and rarely multiple;
Biphasic stromal and epithelial process in which peri-canalicular pattern is most common and lacks leaf-like growth pattern in uniformly hyper cellular stroma. Fibrotic areas may be present.
Lack of atypical features in stroma-like periductal increase in cellularity, stromal overgrowth, cytologic atypia, and mitotic rate>3/HPF;
Frequent epithelial and myoepithelial hyperplasia;
Most patients’ age is 10–20 years with a mean age of 15years.4
Juvenile fibroadenomas may be multiple.4 Giant fibroadenoma is defined as a tumour>500 gms or more than 5cm or replacing at least 80% of the breast. It is more frequently seen in young and black patients. Giant fibroadenoma is of -Adult type and Juvenile type.4, 7 Juvenile type giant fibroadenoma is rare, accounting for 0.5% of all fibroadenomas. Cellular fibroadenoma is a variant of fibroadenoma characterized by diffuse stromal hypercellularity. Giant juvenile fibroadenoma is an uncommon tumour presenting in adolescent females and the exact etiology is not known. Hormonal influences are thought to be contributing factors.7 Excessive estrogen stimulation and/or receptor sensitivity or reduced levels of estrogen antagonist during puberty have been implicated in pathogenesis.7
Bilateral giant juvenile fibroadenomas are extremely rare in pre pubertal girls with very few cases reported in English literature.8, 9, 10, 11, 12, 13, 14, 15
Table 1
|
S. No . |
Author name/Year |
Age |
Clinical features |
|
1 |
Mukhopadhyay M et al.8 2009 |
11yrs. |
Bilateral rapid growing breast lumps, mobile slightly tender and lobulated. Right breast lump of 22 x 20 cm and left breast lump of 18x 16 cm |
|
2 |
Nikumbh DB et al.9 2011 |
12yrs. |
Right breast lump of 15 x 12 and left of 17x15cm, bilateral mobile. |
|
3 |
Goyal S et al.10 2014 |
11yrs. |
Rapidly enlarging right breast lump for three months (16 x13 cm). |
|
4 |
Khan S et al.11 2015 |
10yrs. |
Progressive enlargement of both breasts for 1yr, freely mobile with variable consistency. Right breast of size 6 x 5 cm and left of 10 x15 cm. |
|
5 |
Muthukumaran J et al.12 2015 |
11yrs. |
Girl with enlarging lump of 6 x 5 cm in left breast. |
|
6 |
Beniwal K et al.13 (2 cases); 2015 |
13yrs |
Rapid enlarging lump on left breast forfour months |
|
|
|
11yrs |
Enlarging lump of right breast for two months |
|
7 |
Gaurav et al.14 2015 |
10yrs. |
Rapidly increasing left breast lump of 20x20 cms. |
|
8 |
Nikhil Makkar et al15 2017 |
14yrs. |
Large bilateral mobile lumps of breast. |
|
9 |
Our case; 2020 |
10yrs. |
Rapidly enlarging lump in left breast of 10x8 cms, right breast lump of 8x6 cms. |
The genetic basis of fibroadenoma has not been studied as widely as done for breast carcinoma. Recent studies have shown association of somatic mutations of Exon 2 of the MED 12 gene with benign fibro epithelial lesions of breast.16
The MED 12 gene codes for protein called mediator complex subunit 12 present on chromosome X (Xq13.1), which consists of a group of about 25 proteins that work together to regulate the activity of gene.17 MED12 is important in the transcription regulation of all RNA polymerase II-dependent genes.17 MED 12 forms the kinase/CD8 MODULE which along with CDK8/CDK19, Cyclin C, and MED13 leads to transcriptional repression and acts as positive co- regulator within the p53 transcriptional program.18
Recent study by Lim et al. in 2014 demonstrated there is increased evidence of MED12 exon 2 mutations in genomic drivers of fibroadenoma. First 100 amino acids of exon 2 plays important role in activity of MED12 with Cyclin C. The MED 12 somatic mutation is said to be tumorigenic as it leads to loss of mediator associated CDK activity and overexpression of RAD51B by decreased interaction between MED12 and Cyclin C-CDK8/CDK197.6
The MED12 somatic mutation also has an influence on estrogen signaling as Mediator complex interacts with α and β receptors of estrogen, evidenced by association of MED12 with other estrogen stimulated tumour’s like uterine leiomyoma’s and stromal tumours.6, 16
In a study by DAROOEI et al. The sequence analysis of the MED12 gene using the pairwise sequence alignment tool revealed genetic variations in FA samples in the hotspot region of exon 2/intron 122. It is observed that the codon 44 is the hotspot/highly mutated region of MED12 accounts for (19/22) 86% of observed FA mutations.19 Genetic variations of FA samples consist of 14 unique exonic variations, which include nine SNPs(c.122T>C, c.126A>T, c.127C>G, c.129A>T, c.130G>A, c.130G>C, c.131G>A, c.165G>T, and c.205G>T;) two insertions (c.125_126insATG and c.129_130) and three deletions (c.124_159del c.100_134del and c.171delC,) and six unique intronic mutations (two SNP c.33 +477T>G and 33+429A>C; one insertion c.33+476_477 G three deletions c.33 +423delC, c.33+474_519del 33+429delA).19
c122_ 139 deletion in MED 12 Exon 2 has been reported in this case.
Conclusion
Identifying MED12 gene in fibroadenomas opens the door of understanding the lesser-known genetic basis and genetic abnormalities of highly common lesions such as fibroadenoma and thus leading to development of novel treatment strategies with an aim to target the causative gene mutation and spare the patient of surgical intervention.
It is more so important in cases of multiple and recurrent fibroadenomas, when they are a component of other syndromes to target the MED12 gene with newer drugs and reduce the physical and psychological stress of such young patients due to repetitive surgical procedures.