Introduction
Epithelial-myoepithelial carcinoma (EMCA) is a rare salivary tumor with dual cell population: luminal ductal cells and outer myoepithelial cells. EMCA was initially described by Donath et al.1 and was previously named as adeno-myo-epithelioma, clear cell adenoma or carcinoma. Although high-grade EMCA cases have been rarely reported,2, 3, 4, 5 EMCAs are commonly low-grade tumors and have to be distinguished from pleomorphic adenoma (PA). Infiltrative growth, sharp demarcation from hypocellular hyalinized stroma, retraction (split) artifact between the ductal and abluminal myoepithelial cells are distict characteristics of EMCA. In the year 1991, the World Health Organization recognized EMCA as a distinct entity and subtype of salivary gland adenocarcinoma6 EMC represents <1% of all salivary gland tumors and arises most commonly in the parotid gland, but it has also been described in the submandibular gland, minor salivary glands and palate. There is a female predominance, with a peak occurrence in the seventh decade of life. Clinically, EMCA usually appears as a bulky, slowly growing mass within the parotid gland. Computed tomography (CT) and magnetic resonance appearances are non-specific and the histo-pathological diagnosis may be challenging.7 A more accurate definition of the disease can be achieved by histological and immune-histochemical study. Here we report a case of EMCA which was differently reported in two different occasions and hence proved to be a challenging case.
Case Report
This case is about a 42-year-old gentleman from West Bengal, India, who presented with a painless swelling below angle of mandible on left side. He gave a past history of two surgeries being performed for similar complaints in the same region, which were done in a span of 8 months. Histopathological examination revealed adenoid cystic carcinoma of salivary gland and chronic sialadenitis respectively. He did not receive any other treatment post resection.
Due to recurrence of tumor and varying histopathology reports, the patient was re evaluated in our centre. On examination the swelling was 3x2cm in size, hard in consistency, with skin adherent over the swelling. There were multiple level II cervical lymph nodes palpable on the same side. Repeat FNAC of the swelling showed poorly differentiated adenocarcinoma of submandibular salivary gland. Review of the old histo-pathological slides showed similar results as reported previously. A contrast enhanced CT scan of the neck was done which revealed speculated lesions in the submandibular region 2.4x3x1.8cm inseparable from lateral part of hyoglossus muscle with subcutaneous stranding. Metastatic workup confirmed absent local and distant metastasis.
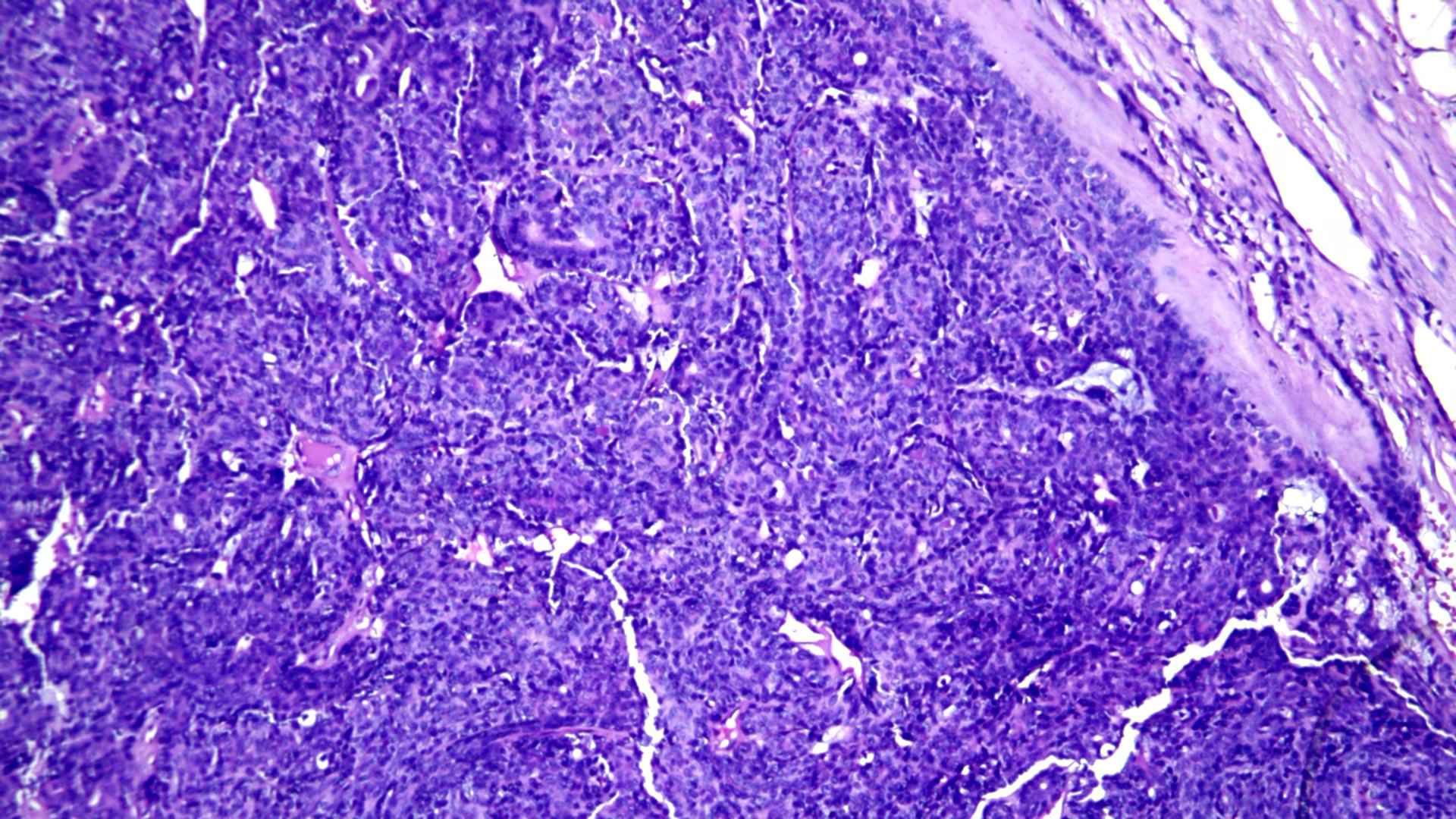
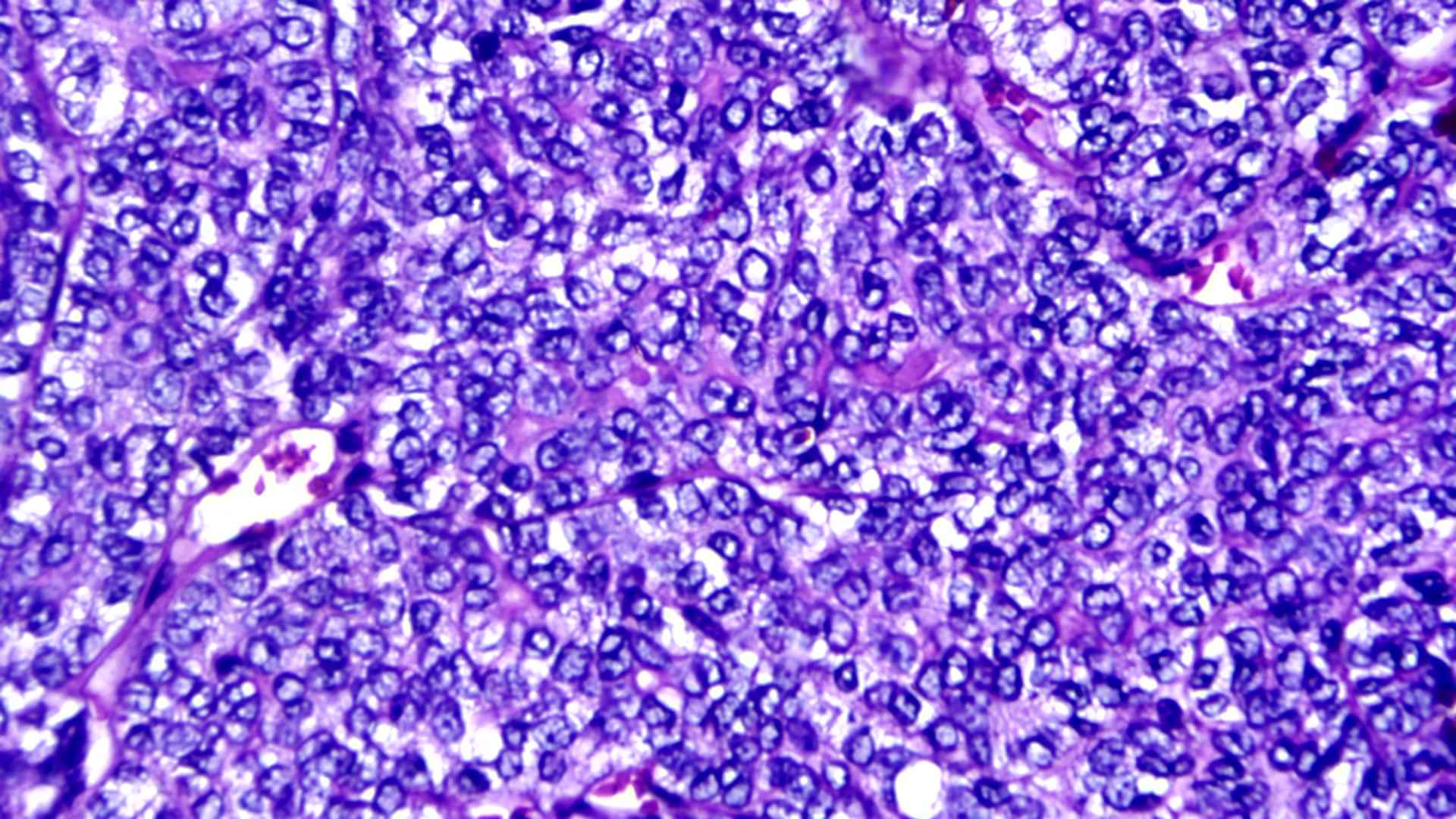
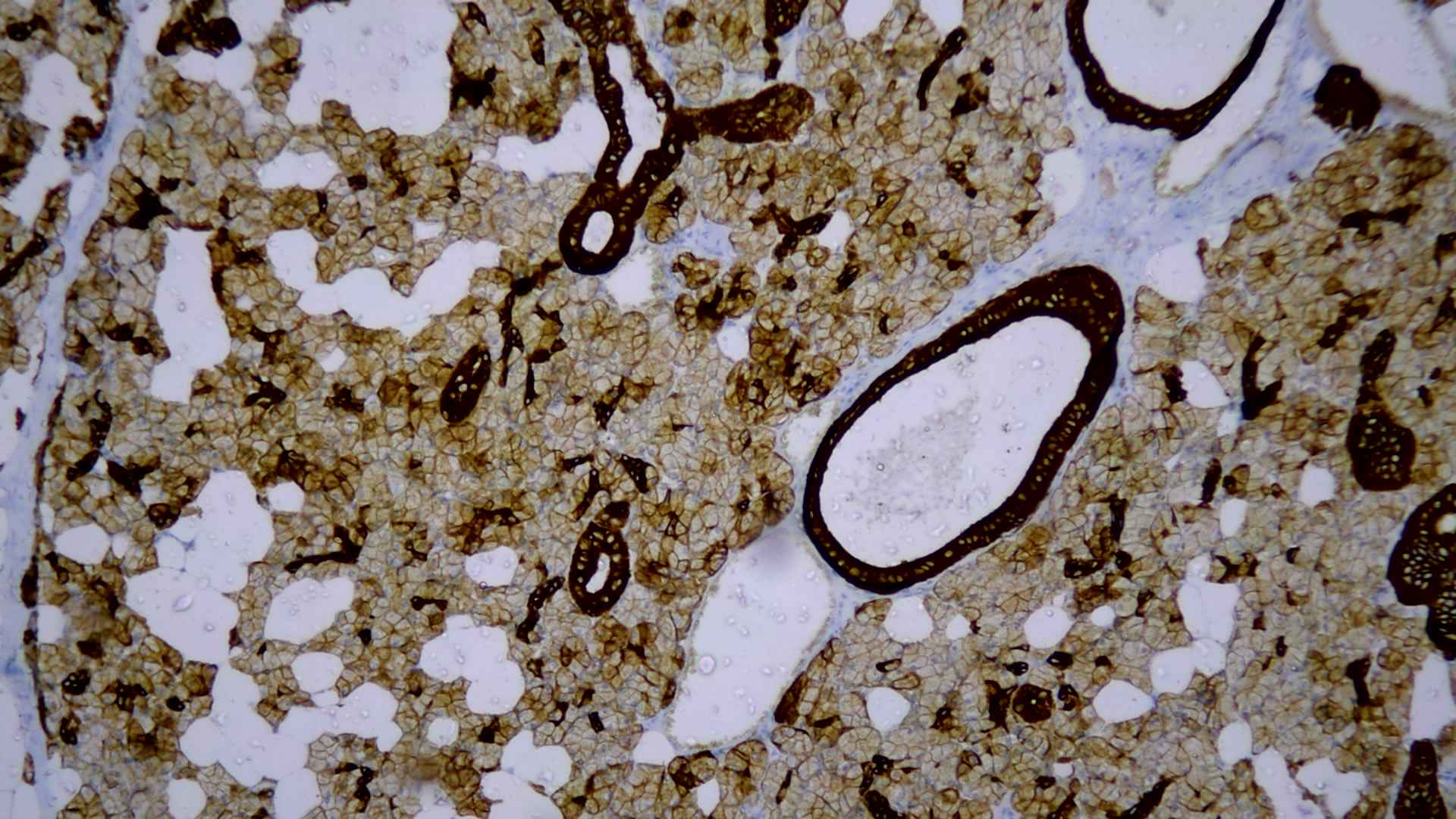
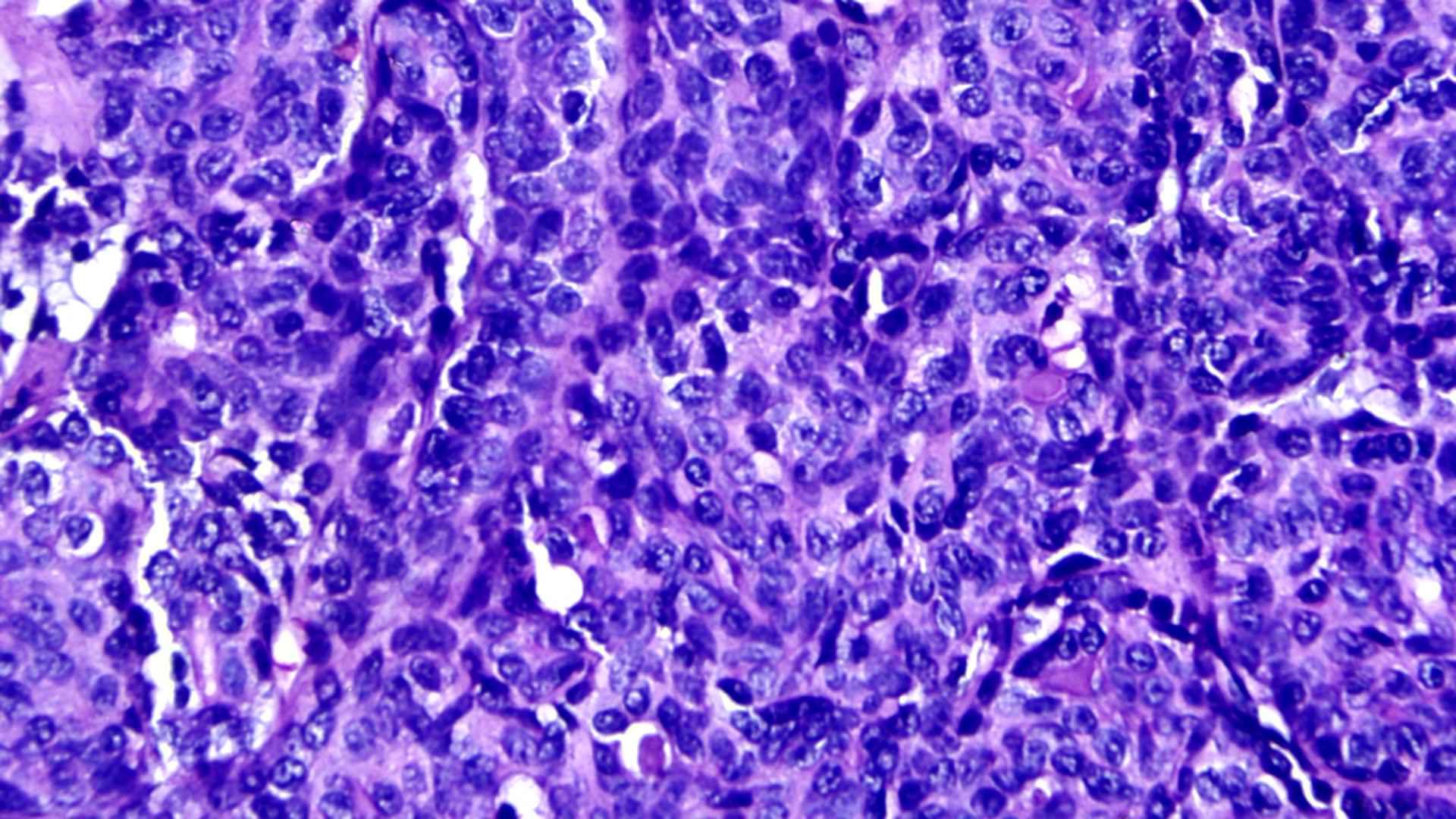
A provisional diagnosis of recurrent adenocarcinoma of the left submandibular gland was made with clinical staging of T2N0M0. The patient underwent left sided modified radical neck dissection type I. Histologically lesion showed an encapsulated cellular neoplasm composed of anatomising tubules and sheets of basaloid cells with scanty stroma in between (Figure 1). Areas showing clusters of clear cells were also noted (Figure 2). Diagnosis of cellular pleomorphic adenoma or basal cell adenoma was given and suggested immune-histo-chemistry (IHC) to confirm. IHC demonstrated both epithelial (CK) and myoepithelial cells (P63) (Figure 3). Glial Fibrillary Acidic Protein (GFAP) was negative; almost all PAs show strong and diffuse expression of GFAP. In contrast, most malignant neoplasm’s that mimic PA on biopsies show only no or only focal expression of GFAP. The presence of strong and diffuse GFAP expression usually favors a benign neoplasm, specifically a PA. Ki-67 was 13% which also favored a malignant neoplasm unlike that of pleomorphic adenoma which shows a low Ki-67 proliferative index (mean 1.6%). IHC showed a biphasic tumor with 12% Ki-67 proliferative index and GFAP positivity, favoring epithelial myo-epithelial carcinoma (Figure 4). There was no evidence of perineural or lympho-vascular spread in biopsy.
The case itself proved to be of a clinical dilemma as all three histo-pathological reports were entirely different from each other. The final report obtained at our centre itself was a rare tumor without well-defined post-operative protocol. Hence, the case was taken up in tumor board discussions, and it was decided to provide post-operative chemo-radiotherapy. He received 30 fractions of external beam radiation and 6 cycles of chemotherapy. The patient is under regular follow up for the last four years and has not shown any recurrence.
Discussion
EMCA is a rare, low-grade malignant neoplasm characterized by a dual cell population of luminal ductal cells surrounded by large, polygonal clear myoepithelial cells. In the data collected by the Armed Forces Institute of Pathology, EMCA constitutes barely 1% of all salivary epithelial neoplasms and nearly 2% of malignant salivary epithelial neoplasms8 EMCA is primarily a tumor of older adults, with a peak incidence in the sixth and seventh decades of life; while this case is a rare one, arising in a younger patient (42 years old).9 The differential diagnosis of EMCAs includes primarily adenoid cystic carcinoma, canalicular and basal cell adenoma, myoepithelioma and myoepithelial carcinoma. Because EMCA is considered to be a low-grade malignant tumor, adequate resection with negative soft-tissue margins is the minimum recommended and necessary therapy. But from our case report, it is evident that inadequate postoperative treatment could lead to recurrence, subjecting the patient to multiple surgeries and related co morbidities. Neck node dissection should be considered in cases of lymph node positivity along with chemotherapy and radiotherapy in patients with highly advanced disease, positive surgical margins, or surgically unresectable disease, although there have been almost no studies of these therapies. Even though our case only met the criteria of recurrence without lymphovascular metastasis, post-operative chemoradiation proved to be highly beneficial. Even though it’s a rare tumor, considering its recurrence rate, whether there is a need to adopt similar treatment protocols for all cases is yet to be concluded.
Acknowledgement
Patient for his co-operation and follow up.
Dr. Christopher Udayan C, MBBS, MD, Dip.RCPath (UK), Consultant Pathologist and Lab Director, Dianova laboratories, Kottayam, Kerala, India for his valuable support and opinions.
Dr. Jophy Varghese, MBBS, MD, Consultant pathologist, DDRC for her slide contributions.