Introduction
Colorectal cancer (CRC) is highly prevalent throughout the world and represents the third most common cancer in men and the second in women worldwide. The incidence rates for CRC have been seen to have a very wide variation in both genders worldwide. At present, India has a comparatively low incidence rate of CRC.1 Population-based time-trend studies show a gradual rise in the incidence of CRC and studies conducted amongst the Indian diaspora in the west show an incidence rate higher than those in India.2, 3, 4, 5 Dietary factors and lifestyles associated with developed countries are important risk factors when it comes to CRC. With India undergoing an economic growth spurt, the burden of CRC in India will likely increase.
One of the many phenomena that have been linked to the pathogenesis of CRC is Microsatellite Instability. Microsatellite instability (MSI), coined by Stephen Thibodeau et al. in 1993, is a term used to denote a hypermutable phenotype caused by the loss of DNA mismatch repair (MMR) activity.6 Microsatellites are stretches of DNA where a single mononucleotide or units of mononucleotides (di-, tri-, tetra-, penta- etc.) are repeated throughout the genome.7 The expansion or contraction of these microsatellites, caused by unrepaired insertions or deletions following an abnormally functioning MMR, gives rise to microsatellite instability.8 Since its discovery in the late 1970s, MSI has been implicated in the pathogenesis of many conditions including, but not limited to, colorectal cancer, gastric cancer, endometrial cancer, ovarian cancer, sebaceous carcinomas and urinary tract cancer.9
Patients with microsatellite instability may have a germ line mutation in one of several MMR (mismatch repair) genes (e.g.: hMLH1, hMSH2, hMSH6 or hPMS2).10 Detection of these defective MMR genes in colorectal carcinomas is important for the detection of Lynch syndrome (Hereditary Non-Polyposis Colorectal Cancer Syndrome - HNPCC), which has clinical implications for treatment of the affected patient and family members as HNPCC is inherited in an autosomal dominant manner.
Testing for MMR mutations in colorectal carcinomas is not a routinely done ancillary study in all except a few top-tier institutes in India. In contrast, screening for mutations in MMR genes in cases of colorectal carcinomas and in colorectal adenomas have been part of the standardized CAP protocol, followed by pathologists all around the world, for the past decade.
Through this study we aim to evaluate via immunohistochemistry, (viz. the markers MSH2, MSH6 and MLH1), the frequency of MMR mutations (and thereby, microsatellite instability) in cases of CRC seen in a tertiary institute in Mangalore.
Materials and Methods
This study was conducted prospectively on cases of colorectal carcinoma received in the Department of Pathology, Yenepoya Medical College. As per the guidelines and clearances received from the Institutional Ethical Committee, the study was conducted on specimens received during the time periods of January to December 2016 and May to October 2017.
The sample constituted of 40 consecutive cases of colorectal carcinoma fulfilling the inclusion criteria, received in the time period mentioned above.
The inclusion criteria for the study were as follows:
Cases of primary colorectal carcinoma
Surgical resected cases of colorectal carcinomas.
Cases where prior chemotherapy or radiotherapy has not been given.
The exclusion criteria for the study were as follows:
Metastatic carcinoma to the colorectum
Biopsy specimens
Post chemo or radiotherapy patients.
All cases of colorectal adenomas without frank co-existing malignancy.
All specimens were received in the department immediately following surgery and were then kept for overnight fixation in 10% neutral buffered formalin. The next day, the specimen was cut open and kept for further fixation overnight.
Grossing was performed according to the according to College of American Pathologist (CAP) protocol. The size (3 dimensions in cm), site, and evidence of macroscopic tumour perforation were specially noted.
Left and right sided tumours were determined as follows:11
Patients with cancer located in the right side of the colon were those with tumour in the cecum and/or ascending colon and/or transverse colon.
Patients with cancer located in the left side of the colon were those with tumour in the splenic flexure, descending colon, sigmoid colon or rectum.
The tissue bits given were processed overnight using a Leica TP1020 Semi-Enclosed Benchtop Tissue Processor (Leica Biosystems, Wetzlar, Germany). The processed tissue was then embedded in Surgipath© Paraplast Paraffin (Leica Biosystems, Wetzlar, Germany) at a HistoCore Arcadia H - Heated Paraffin Embedding Station (Leica Biosystems, Wetzlar, Germany). After cooling the blocks on a HistoCore Arcadia C - Cold Plate (Leica Biosystems, Wetzlar, Germany), 4µm thin sections were cut from the blocks using Leica RM2245 Semi-Automated Rotary Microtome (Leica Biosystems, Wetzlar, Germany).
The sections were stained with Hematoxylin and Eosin on a Leica ST5010 Autostainer XL (Leica Biosystems, Wetzlar, Germany), and mounted using DPX Mountant for Histology (Sigma-Aldrich, St. Louis, Missouri, USA) on Blue Star© frosted micro slides (Blue Star© Slides, Mumbai, India).
The expression of MMR proteins (which was earlier validated on normal as well as tumour tissue) was evaluated using IHC markers for MLH1, MSH2, MSH6 and PMS2.
Hematoxylin and eosin slides of each case were first screened for an appropriate representative tumour section. IHC was then performed on this section.
The antibodies, the type of antigen represented, clones and dilutions are listed in Table 1.
Table 1
Details of IHC markers used
All the markers were stained manually.
Changes in protein expression by IHC were evaluated in stained sections by two pathologists. MMR protein expression was considered negative when all the tumour cell nuclei failed to react with antibody and considered positive by the presence of intact nuclear staining within the tumour regardless of its intensity or the number of positive nuclei. Cytoplasmic staining without nuclear staining was also considered negative. All the tumour sections selected had adjacent normal tissue (non-neoplastic colonic mucosa, stromal cells, infiltrating lymphocytes or the centres of lymphoid follicles) for eliciting intact nuclear staining as positive internal control for positive staining and a negative control was carried out without the primary antibody.
Data was entered into Microsoft Excel 2016 worksheets and then further statistical analyses were performed on SPSS version 23 (IBM).
Results
The present study conducted in the Department of Pathology, Yenepoya Medical College, Mangalore, included a total of 40 resected specimens of colorectal carcinoma.
The youngest patient in this study was 18 years old while the oldest was 78 years. The median age at which the resection was performed was 51 years. Out of the 40 cases, the most number of cases belonged to the 50-59 years age group (n=12, 30%), followed by equal number of cases in 30-39 years and 60-69 years age group (n=7, 17.5%). Males were found to be more than females with a male to female ratio of 1.1:1. The rectosigmoid junction was the commonest site of occurrence of colorectal carcinoma (n=7, 17.5%). The majority of the tumours presented as left sided tumours (n = 22, 55%) and the remaining as right sided tumours (n = 18, 45%).
The tumour sizes in the study ranged from 1.5 to 11 cm in greatest dimension. The overall mean tumour size was 5.3 cm. The mean tumour size for left sided tumours was 5.16 cms and the mean size for right sided tumours was 5.47 cms. On classifying the 40 cases of resected colorectal carcinoma according to the latest WHO Classification, adenocarcinoma was the most common histological subtype accounting for 90% of cases (n = 36). Mucinous carcinoma was seen in 10%. No other histological types were encountered in the current study. The grading of the colorectal carcinomas was done as per the WHO guidelines. According to this grading system, most of the tumours were moderately differentiated - G2 (n = 29, 72.5%).
All 40 cases were stained for four MMR proteins viz. MSH2, MSH6, MLH1 and PMS2. Of these, only 3 cases (7.5%) showed negative IHC staining of tumour cells for mismatch repair proteins.
The first case was a 68 year-old female with a moderately differentiated (low grade) adenocarcinoma, seen arising from the caecum and measuring 7cm in greatest dimension. No intratumoural lymphocytes, peritumoural lymphocytic reaction, dirty necrosis, lymph-vascular or perineural invasion was seen. A mucinous component was noted. The case had a TNM staging of pT2N0Mx – Stage I. On IHC, a concurrent loss of MSH2 and MSH6 was seen with a strongly positive intact nuclear staining of MLH1 and PMS2 (Figure 1). The second case was a 54 year-old male with a moderately differentiated (low grade) adenocarcinoma, seen arising from the caecum and measuring 5cm in greatest dimension. No dirty necrosis, lymph-vascular or perineural invasion were seen. A mucinous component was noted along the presence of intratumoural lymphocytes and a peritumoural lymphocytic reaction. The case had a TNM staging of pT2N0Mx – Stage I. On IHC, a concurrent loss of MLH1 and PMS2 was seen with a strongly positive intact nuclear staining of MSH2 and MSH6 (Figure 2). The final case was a 50 year-old male with a well differentiated (low grade) adenocarcinoma, seen arising from the descending colon and measuring 5cm in the greatest dimension. No lymphovascular or perineural invasion were seen. A mucinous component was noted along the presence of intratumoural lymphocytes, peritumoural lymphocytic reaction and dirty necrosis. The case had a TNM staging of pT2N0Mx – Stage I. On IHC, an isolated loss of MSH6 was seen with a strongly positive intact nuclear staining of MSH2 and a weakly positive intact nuclear staining with occasional cytoplasmic staining (no significance is attributed to cytoplasmic staining) of MLH1 and PMS2.
Figure 1
Concurrent loss of MSH2 & MSH6; A: MSH2 - Tumour shows a complete loss of nuclear staining, whereas the internal control i.e the stromal cells and lymphocytes in the stroma stain strongly positive; B: MSH6 - Tumour shows a complete loss of nuclear staining, whereas the internal control i.e. the stromal cells and lymphocytes in the stroma stain strongly positive; C: MLH1 – Tumour cells show strongly positive nuclear staining; D: PMS2 – Tumour cells show strongly positive nuclear staining
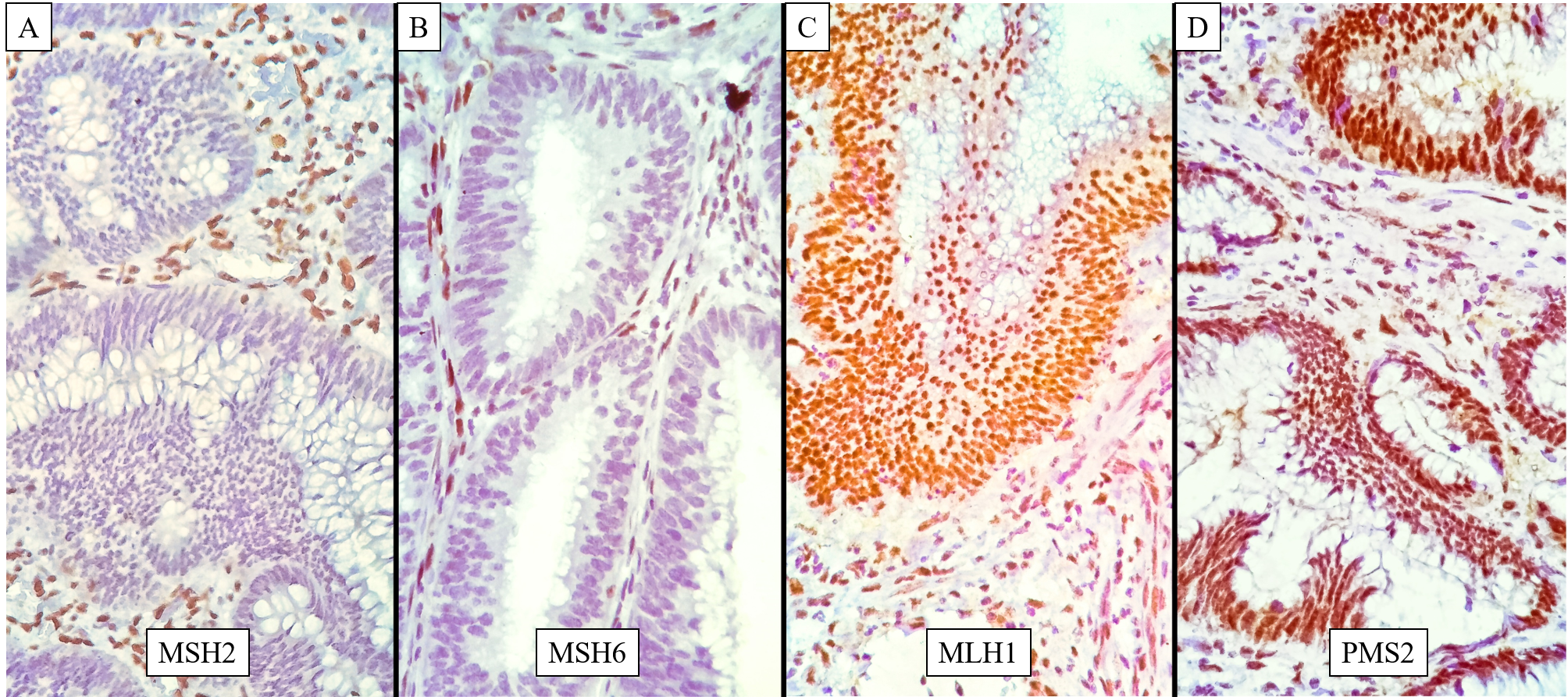
Figure 2
Concurrent loss of MLH1 & PMS2; A: MSH2 - Tumour cells show strongly positive nuclear staining; B: MSH6 - Tumour cells show strongly positive nuclear staining with occasional weak cytoplasmic staining; C: MLH1 – Tumour shows a complete loss of nuclear staining, whereas the internal control i.e. the stromal cells and lymphocytes in the stroma stain positive along with patchy cytoplasmic staining which is considered insignificant; D: PMS2 – Tumour shows a complete loss of nuclear staining, whereas the internal control i.e. the stromal cells and lymphocytes in the stroma stain strongly positive.

Figure 3
Isolated loss of MSH6; A: MSH2 - Tumour shows a moderate to weak nuclear staining – considered as intact nuclear expression of MSH2; B: MSH6 - Tumour shows a complete loss of nuclear staining (black arrow), whereas the internal control i.e. the stromal cells and lymphocytes in the stroma stain positive (red arrows); C: MLH1 – Tumour cells show weakly positive nuclear staining - considered as intact nuclear expression of MLH1; D: PMS2 – Tumour cells show moderate to weak nuclear staining – considered as intact nuclear expression of PMS2

Discussion
CRC is a malignancy affecting thousands of people in India every year. The goal of cancer treatment is to improve the quality of life and overall survival. A better understanding of the biology behind cancer can open new insights into creating targeted approaches to treatment. Many different pathways have been implicated in the pathogenesis of CRC with the highest impact amongst these pathways being those that are genetically inherited. Mutation in a mismatch repair gene(s) is one such phenomenon which can lead to cancer exhibiting microsatellite instability.
In this study, tumours with defective MMR proteins constituted just 7.5% of the total cases, a stark contrast to most studies conducted in India. All the other IHC studies of microsatellite instability in India, using MMR markers, have reported lack of nuclear stain in proportions varying widely from 1.01% to 41.90% (Table 2).8, 12, 13, 14, 15, 16
Table 2
Comparison of MMR loss among IHC studies in India
|
Study |
Sample Size |
Country |
dMMR / MSI |
|
Current Study |
40 |
India |
7.50% |
|
Gandhi et al12 |
62 |
India |
41.90% |
|
Ostwal et al14 |
296 |
India |
1.01% |
|
Paulose et al15 |
195 |
India |
27.10% |
|
Nayak et al16 |
231 |
India |
22.94% |
|
Dubey et al13 |
45 |
India |
22.20% |
|
Pandey et al8 |
46 |
India |
15.70% |
Similar results were also seen in the studies of Chang et al. (2010), Goshayeshi et al. (2017) and Watson et al. (2007) which reported values of 7.3%, 8.7% and 9.62%.17, 18, 19 On the opposite side of the spectrum, were the results of Hashmi et al (2017) and Hall et al (2010) who reported relatively higher values of 34% and 30.20% respectively.20, 21
As seen above, the percentage of MMR deficient cases in this present study is comparatively low (except when compared with the results of Ostwal et al.). This may be due to the relatively small sample size, difference of ethnicity and less probability of Lynch syndrome. It is also possible that the actual percentage of cases showing mutation in the MMR genes is much higher, but the inactivation of the 2nd allele of the MMR gene may not have resulted in a loss of expression detectable by IHC.22, 23
Out of the three cases with MMR loss, one case showed isolated MSH6 loss, one case showed a concurrent loss of MSH2 and MSH6, while one case showed concurrent loss of MLH1 & PMS2.
Pandey et al. (2007) studied 46 cases of CRC using only 2 IHC markers i.e. MLH1 & MSH2, and out of the 46 cases, 8 patients had one of the mismatch repair proteins missing, out of which 7 lacked MLH1 & the remaining one case was lacking MSH2.8
Dubey et al (2016) examined 45 cases using 4 IHC markers and found that 10 cases showed a loss of MMR protein expression. In this study, all cases showed a concurrent loss of MLH1 & PMS2; no other staining pattern was noted.13
Gandhi et al (2018) also used 4 IHC markers on 62 cases of stage II CRC, out of which 26 (41.9%) demonstrated a loss of MMR proteins. Out of the cases with MMR loss, 38.4% showed a concurrent loss of MLH1 & PMS2, 30.7% showed a concurrent loss of MSH2 & MSH6, 26.9% showed an isolated loss of PMS2 and 3.8% showed an isolated loss of MSH6.12
Ostwal et al (2019) assessed 296 patients using IHC and only 3 patients showed dMMR status – one has dual loss of MLH1 and PMS2, the second had dual loss of MLH1 and MSH6 expression and the third had dual loss of MSH2 and MSH6 expression.14
Nayak et al (2018) examined 231 cases, out of which 13.9% had dual loss of MLH1 and PMS2, 7.4% showed dual loss of MSH2 and MSH6 and only 1.73% showed isolated PMS2 loss.16
Given that percentage of MMR protein loss in the present study is comparatively low and that all three cases show disparate staining patterns, no comparison or contrast can be made with other studies regarding the predominance of any one or more MMR loss patterns. When interpreting IHC patterns of MMR protein loss, one must remember that PMS2 and MSH6 form functional dimers with MLH1 and MSH2 respectively with expression being dependent on MLH1 / MSH2. Loss of expression of MSH2 is most often associated with a loss of expression of MSH6; a pattern highly suggestive of a MSH2 germ-line mutation. Loss of expression of MLH1 expression is often seen hand-in-hand with loss of PMS2. Concurrent MLH1 & PMS2 loss usually results from either an MLH1 germ-line mutation or an acquired somatic hypermethylation of the promoter region of the MLH1 gene. Isolated loss of PMS2 and MSH6 are generally associated with germ-line mutations of MSH6 and PMS2.24, 25 EPCAM deletion leading to MSH2 epimutation can also cause isolated loss of MSH2 expression.26 Nucleolar staining or complete loss of MSH6 staining has been described in CRC cases with prior radiation or chemotherapy, and a significant reduction of MSH6 staining has been described in a small percentage of colorectal carcinomas with somatic mutations of the coding region microsatellites of the MSH6 gene in MLH1/PMS2-deficient carcinomas.27, 28
Conclusion
Data regarding mutations in MMR genes and microsatellite instability in colorectal carcinoma, in a South Indian population, is scant. Given the genetic impact that defective MMR mechanisms have with regard to hereditary colorectal cancer syndromes, a detailed profile of this phenomenon and of CRC in general, is imperative in any population where the incidence of colorectal cancer is on the rise.
The present study revealed that a subset of colorectal carcinomas in a Mangalorean population do show MMR mutations and by association, MSI. However, the proportion of colorectal carcinomas exhibiting a defective MMR mechanism, thereby implicating microsatellite instability, is relatively low. It is much lower than other studies done in India and outside.
The low percentage seen may be due to the relatively small sample size, difference of ethnicity and less probability of Lynch syndrome. Also, as this study demonstrated MSI using only IHC, it is possible that the actual rate of MSI positive CRCs, i.e. Lynch syndrome, might be higher. There may be persistence of MMR expression if the inactivation of the second allele of the MMR gene does not result in loss of expression detectable by IHC.
A study with a larger patient cohort and using advanced ancillary techniques such as PCR & gene sequencing is indicated for further in-depth analysis of MSI positive CRCs in and around Mangalore.
Disclosure
This study was done as part of the MD Pathology dissertation from Yenepoya Medical College, Mangalore, Karnataka – 575018
This study was presented as an oral paper at the 46th Annual State Conference of the Karnataka Chapter of the Indian Association of Pathologists and Microbiologists, held at S.N Medical College, Bagalkot on 21st September, 2019.
