Introduction
Ovarian cancer is a commonly diagnosed and particularly deadly gynaecologic malignancy world-wide.1 The majority of cases are diagnosed at advanced stage III or IV.2 Early detection of malignancy will aid to decrease the morbidity and mortality. Increased knowledge of aetiology and pathology of ovarian carcinogenesis will enhance the researches in this field. The newer concept of ovarian carcinogenesis is two pathway model proposed by Shih and Kurman.3 Type I tumors include non serous ovarian tumors and low-grade serous carcinomas. Type II tumors are composed of high-grade serous carcinomas. Type I tumors arises from benign extra ovarian lesions that implant on the ovary and later undergo malignant transformation, whereas many type II carcinomas develop from intraepithelial carcinomas in the fallopian tube and disseminate as carcinomas that involve the ovary and extra ovarian sites. The precursor lesions or earliest histopathological changes in tubal epithelium are defined as SCOUT (Secretory Cell OUT growth) and STIC (Serous Tubal Intraepithelial Carcinoma).4
The fallopian tube epithelium (FTE) is a columnar cell layer composed of two specialized cell types-secretory and ciliated cells. In type II tumors fimbrial regions of FTE exhibit a shift towards the secretory phenotype, with complete loss of ciliated cells and the acquisition of proliferative capacity which is known as SCOUT.5 The significance of SCOUTs lies in the fact that, they are a visible and evaluable precursor entity, the study of which might yield a greater understanding of both pelvic epithelial carcinogenesis and the tubal cell populations that is involved in carcinogenesis. SCOUT lesions later progresses to STIC (Serous Tubal Intraepithelial Carcinoma).6 which is characterised by loss of polarity, nuclear enlargement, hyperchromasia and increased mitotic activity. These histopathological changes are highlighted by p53 immunostaining. Normal fallopian tubes show wild-type staining pattern with p53 (negative TP53 mutation) which is defined as weak, patchy nuclear staining.7 TP53 gene mutation either missense or nonsense is characterised by aberrant p53 immunohistochemical expression. Intense p53 nuclear expression of at least 12 continuous fallopian epithelial cells are defined as p53 signature and is considered as missense mutation.5 Complete absence of p53 immunoexpression is commonly associated with a TP53 non sense mutation.6 The latter two patterns are together called aberrant expression patterns.6 p53 signatures and STICs, when concurrent, share evidence of DNA damage and exhibit identical TP53 mutations, indicating a common origin.7 In our study benign, borderline and malignant serous tumors of ovary and their fallopian tube epithelium are studied. This study compared the p53 expression pattern of serous tumors and their fallopian tubes to explore their relationships
The implications of this new paradigm of ovarian carcinogenesis for researchers, clinicians and women are significant. For women who are BRCA positive or at risk of high-grade serous carcinoma, opportunistic salpingectomy can be considered as a primary prevention of ovarian carcinoma. In general population also removal of fallopian tubes in females undergoing pelvic surgery for other indications or as a sterilization procedure, would improve quality of life & overall survival while still reducing risk of ovarian carcinoma.8
Materials and Methods
The present study was a single center cross sectional study
A total of 90 serous tumors of ovary including benign, borderline, malignant cases and their fallopian tubes were examined using 4 micrometer thin H&E stained sections. Clinical details were collected from medical records. Serous tumors of ovary were categorized into benign, borderline, low-grade and high-grade carcinomas. Their fallopian tubes were examined using Sectioning and Extensively Examining the FIMbriated end (SEE-FIM) protocol9 and examined for presence of SCOUT and STIC lesions.
Secretory cell out growth (SCOUT)
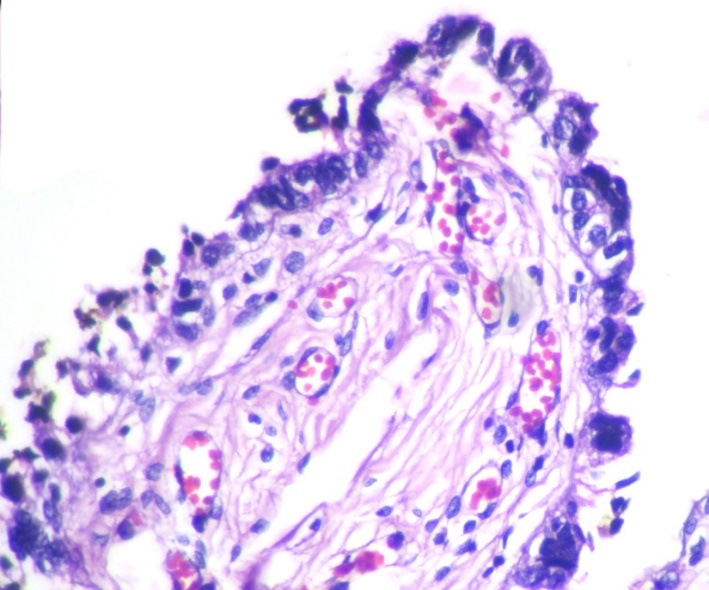
SCOUT was defined as a linear stretch of continuous 30 or more secretory cells in the tubal epithelium with no intervening ciliated cells. 5
Serous tubal intraepithelial carcinoma (STIC) 6
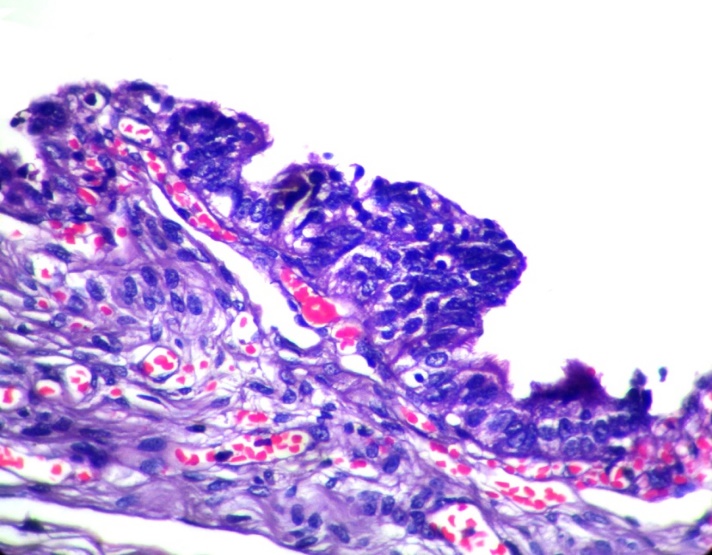
STIC was defined as loss of epithelial polarity, cellular dedifferentiation, mitotic activity, nuclear pleomorphism and nucleolar prominence in tubal epithelium.
Relevant sections of ovarian tumor and fallopian tubes were stained for p53 immunostaining using DAKO Flex monoclonal mouse anti-human p53 protein clone DO-7 over Formalin fixed paraffin embedded sections which were mounted on poly-L-Lysine coated slides. Further sections were deparaffinized and pressure-cooking method was used for antigen retrieval. After that endogenous peroxidase blocking was performed. Then, incubation with monoclonal primary antibody was done for 30 minutes. Secondary antibody conjugated with horseradish peroxidase enzyme was applied for 30 minutes. Thereafter, freshly prepared di-amino benzidine (DAB) was applied for 10 minutes. Finally, hematoxylin was used for counterstaining and slides were then dehydrated and mounted. P53 expression patterns were recorded. Scattered nuclear positivity in less than 10% 0f cells were recorded as wild type pattern. Strong to moderate nuclear positivity in continuous 12 or more cells was recorded as p53 signature. Complete absence of staining was separately noted. Latter two patterns were together called as aberrant pattern of p53 expression.
Analysis of data
All variables were expressed in frequency and percentages. Chi square test was used to obtain the association of study variables with groups. Data was entered in excel sheet and analyzed by using IBM Statistical Package for Social Sciences (SPSS) version 20. The p value <0.05 was considered as statistically significant.
Results
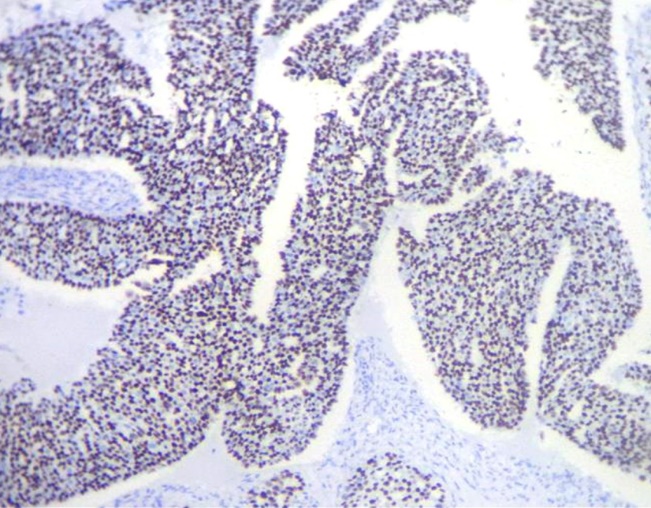
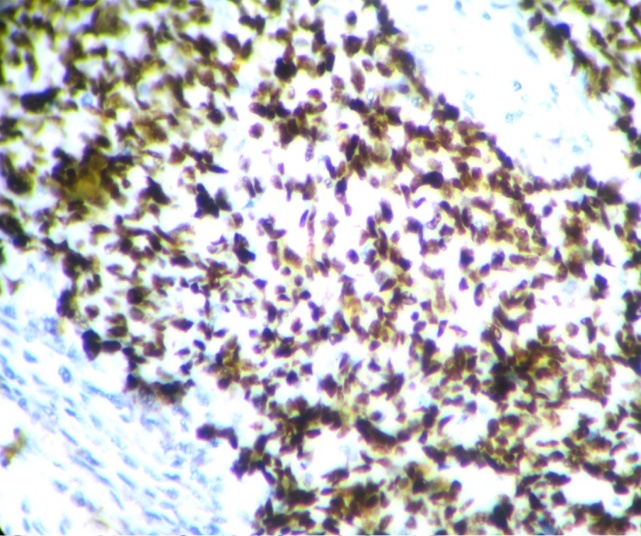
The youngest patient in the study was 18 years and the oldest patient was 83 years old, with a mean age of 47.7years. Out of the 90cases of serous tumors include 61 benign, 6 borderline, 11 low grade and 12 high grade carcinomas.43.3% of fallopian tubes showed normal histology. Most common histopathologic change in fimbriae was SCOUT (54.4%) which is present in all categories of serous tumors. Even though 59% of SCOUT(fig-1,2) lesions were distributed in benign serous tumors, 90.9% of low grade tumors and 83.3% of high grade tumors showed SCOUT.STIC(fig-3,4) lesions contributed 11% of fimbrial pathology.8 out of 11 STICs were present in high grade tumors. In other words, 66.6% of high- grade tumors (8 out of 12) showed STIC whereas it was 16.6%in borderline and 18.1% in low- grade tumors. Benign tumors were negative for STIC. (Table 1)
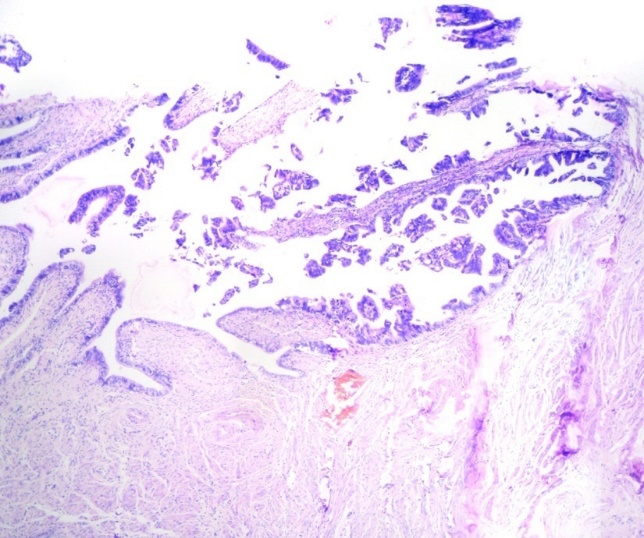
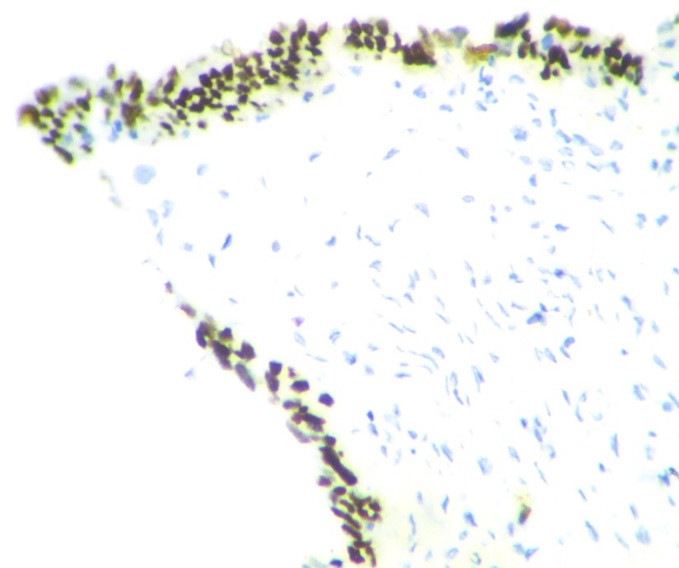
p53 expression was significantly high in high grade serous carcinoma(fig-5,6) (p value <0.001). None of the benign serous tumors showed strong p53 expression. One case of borderline serous tumor, 2 out of 11 low grade and 6 out of 12 high grade serous carcinoma showed strong p53 expression. 42% (5 out of 12) high grade serous carcinoma showed complete absence of p53 staining (Table 2).
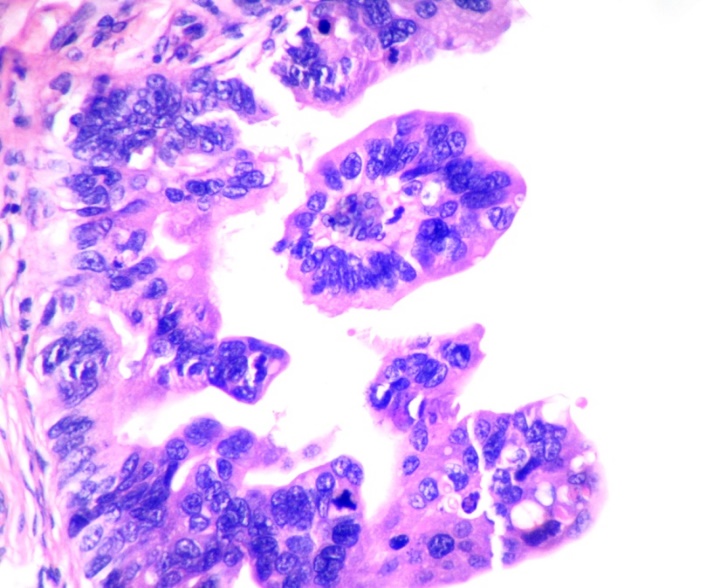
Only 22.4% of SCOUT showed p53 signature.(fig-7) None of the STICs showed wild type pattern. They showed either p53 signature or absent staining pattern. (Table 3)
Distribution of p53 signature in SCOUTs and STICs according to grade of tumor showed significant association with high grade tumors.50% of high-grade tumors showed p53 signature and 41.7% showed absent staining pattern. p53 signature in benign, borderline and low grade tumors constitute 4.6%, 16.7% and 18.2% respectively. (Table 4)
Table 1
Table 2
Table 3
Table 4
Discussion
Ovarian cancer has emerged as one of the most common malignancies affecting women in India and remains the deadliest form of gynaecological malignancy. 10 Unfortunately, high grade serous ovarian carcinomas (HGSOC) carry a poor prognosis, representing over 70% of all epithelial ovarian cancer deaths. 11 Recent researches of epithelial ovarian cancers using molecular and genetic methods have led to a paradigm shift in the classification of this disease via the introduction of the dualistic model of ovarian carcinogenesis.(type 1 and type 2 tumors). This model was first proposed by Kurman and Shih in 2004 and was officially recognized in 2014 by the WHO in their updated classification guidelines for tumours of the female reproductive organs. 12 The Type 1 neoplasms progress from pre-malignant or borderline lesions and display oncogenic alterations in cellular signalling pathways such as RAS-MAPK and PI3K-AKT. They are genomically stable and p53 wild type. From a clinical perspective, these tumors grow in an indolent fashion and when confined to the ovary they have an excellent prognosis. This category includes low-grade serous, clear-cell, mucinous and transitional cell (Brenner) subtypes). 12
By contrast, the Type 2 category is characterized by more aggressive pattern of disease behavior. Tumors develop rapidly and usually are disseminated widely at the time of presentation, resulting in poor overall prognosis.12 These tumors arise from p53mutated fallopian fimbrial epithelium as evidenced by studies done on prophylactic salpingo oophorectomy cases from BRCA positive populations
Lessons from BRCA population
Inherited mutations in BRCA1 or BRCA2 are associated with familial ovarian and breast cancer syndromes and account for about 11–15% of ovarian carcinomas.13 In developed countries, many women with germline BRCA mutations (BRCA+) prefer to undergo bilateral salpingo - oophorectomy for fear of developing ovarian carcinoma. Studies have reported that high incidence (50%) of epithelial dysplasia and occult tubal cancers (0.9-17%) were present in fimbrial region of fallopian tubes of BRCA+ women.14, 15 Those ovaries are thoroughly examined for evidence of occult cancer. Until recently, the fallopian tubes were not closely examined following such surgeries, and thus, early-stage tubal cancers were rarely detected and severely under reported in BRCA+ patients.
The proposed histopathological precursor lesions in fallopian tubes were defined as SCOUTs and STICs. These are highlighted by p53 immunostaining.
Missense mutations in TP53 typically correlate with positive staining due to the mutant protein accumulation. If, however, the gene contains a nonsense mutation, then the resultant truncated form of the protein might not be detectable by the antibody. In that case, the staining would be almost totally negative. In other words over expression of p53 and complete absence of staining are consistent with p53 mutations which require p53 gene sequencing.
This study mainly focuses on the type 2 pathway of tumorigenesis. In our study, p53 has been found to be low or weakly positive in benign and borderline and low grade malignant tumors whereas it is consistently strongly positive in high grade serous carcinomas. A published study by Sallum etal also showed significant p53 expression in high grade tumors.16
In our study 42.6%of benign, 50% of borderline 90.9% of low grade and 83.3%of high grade serous tumors displayed SCOUT. It therefore means SCOUT is seen in all categories of serous tumors. Nearly half of the benign tumors (43%) and borderline tumors (50%) showed SCOUT whereas, more than 80% of malignant tumors displayed SCOUT. In a previous case study, the investigators detected SCOUTs in 12%, 18%, and 83% of the fallopian tubes from women who received salpingectomy for non-malignancy-related reasons, healthy, BRCA-positive women, and women with ovarian HGSC, respectively.5 Present study also go along with their findings.
So, it can be assumed that SCOUT cannot be considered as specific feature of either group instead significant majority of malignant tumors had shown SCOUT. So, SCOUT may be regarded as being an index to fimbrial irritation or injury rather than being an index of malignancy.
None of the benign tumors showed STIC whereas 66.6% of high grade tumors displayed STIC. Among the STICs 72.7%were in high grade carcinomas &18.2% were low grade carcinomas. STIC is more consistently associated with high grade carcinoma. So STIC may be considered as a better index of high grade serous carcinoma than SCOUT. Our results are comparable with other studies.
Table 5
|
Study |
High grade tumors |
STIC |
|
Kindelberger et al 17 |
30 |
66% |
|
Przybycin et al18 |
41 |
59% |
|
Daichi Maeda et al19 |
15 |
47% |
|
Present study |
12 |
66.6% |
None of the benign tumors showed p53 over expression. 16.7% of borderline tumors and 18.2% of low grade carcinoma were presented with p53 signature. 58% of high-grade carcinomas expressed p53 signature. 41.7% of the high grade tumors showed complete absence of staining. So significant cases of high grade carcinoma (11 out of 12) pointed towards p53 mutation either as over expression or as complete absence of staining which have to be confirmed by p53 sequencing studies. (p value<0.001)
Analysis of p53 expression patterns in SCOUT and STIC lesions showed wild type pattern in 77.6% of SCOUT lesions. All STIC lesions showed either over expression of p53 or complete absence of staining pointing towards p53 mutation. p53 positive SCOUTS and STICs were significantly distributed in high grade tumors.
Summary and Conclusions
The hypothesis of our study was that the origin and evolution of high-grade ovarian carcinoma could be from fallopian fimbrial epithelium through p53 mutation. The fallopian fimbrial epithelium of all serous tumors were studied with special attention being given to SCOUT, STIC and p53 expression pattern. We found in our study that a significant proportion of high-grade carcinoma harbour STIC and majority of them showed p53 aberrant pattern of expression. Strong p53 expression was also found in majority of high grade serous carcinomas.
Among all the serous tumors only a portion of cases had SCOUT. But among SCOUTS only 22.4% showed p53 signature. Majority of p53 aberrant pattern is contributed by STIC which is more seen in high grade carcinoma. Significant number of high- grade tumors also showed p53 expression. Hence it can be presumed that SCOUT is an initial event, and a subset of which develop mutation resulting p53 signature, later progressing through p53 positive STICs which have the ability to spread rapidly, moving from fimbria to ovarian surface or exfoliating into peritoneal cavity leading to high grade serous carcinoma in these sites.