Introduction
Crescentic Glomerulonephritis (CGN) is characterized by the presence of extensive glomerular crescents in more than 50% of the glomeruli. It presents with rapidly progressive Glomerulonephritis (RPGN). It could be primary and secondary. CGN is classified into 5 types (Parmar 2017).1
Type I – It shows linear deposits of immunoglobulins IgG along the glomerular basement membrane (Anti GBM disease).
Type II – It shows granular deposition of immunoglobulins and complement (Immune complex mediated disease).
Type III – Few or no immune deposit in glomeruli (Pauci-immune). It is antineutrophilic cytoplasmic antibody (ANCA) associated with small vessel vasculitis.
Type IV – Combination of Type I and Type III
Type V – ANCA, immune complex and anti GBM Ab negative (pauci-immune renal vasculitis).
Pauci-immune crescentic glomerulonephritis (PICGN) accounts for 80% of the cases of rapidly progressive GN.2, 3, 4 Majority of the cases of PICGN are due to primary systemic small vessel vasculitis e.g., GPA, MPA or renal limited vasculitis (RLV).5 ANCA is present in 80% – 90% of the patients with PICGN. Patients with both ANCA associated GN usually present with rapidly progressive GN with haematuria, proteinuria and elevated serum creatinine.6 Histopathologically, these patients have necrotising GN and extensive crescents.7 A small subset of patients have subacute disease and on renal biopsy have glomerular sclerosis either alone or accompanied by focal active disease with necrosis and crescents.8 In most of the patients, PICGN is a component of systemic small vessel vasculitis.
In 2010 International Vasculitis working study group9 have classified ANCA mediated CGN into 4 types which predict the survival rate of patients. This includes
Focal: > 50% normal glomeruli are present.
Crescentic: > 50% glomeruli with cellular crescents.
Mixed: < 50% normal glomeruli, <50% crescentic, <50% globally sclerotic glomeruli.
Sclerotic: > 50% globally sclerotic glomeruli.
Crescentic and mixed group have the worst prognosis when <25% of the normal glomeruli are present.10
CGN is very commonly seen in our country but very few studies are available in India. The aim of study is to find out various subtypes of CGN, and prevalence of ANCA and its subtypes in PICGN.
Materials and Methods
The total 50 cases of CGN were studied between the period of February 2016 to July 2017. Cases were taken from the Department of Nephrology & Dermatology. A detail clinical history was taken and 5ml blood in plain vial was taken after consent of the patients for immunological tests like ANCA, ANA, ds DNA Ab and anti GBM Ab.
Only kidney biopsy proved cases of CGN were taken. Histopatholoically it was classified first according to Parmar 2017.1 classification. PICGN was further classified into 4 groups depending upon International classification proposed by Berden et al 2010.9
Kidney biopsy were preserved in 10% buffered formalin. Paraffin blocks were prepared and sections were stained with Haematoxylin and Eosin (H&E), Periodic Acid Schiff (PAS) and Acid Fuchsin Orange G (AFOG) stains as described by Zollinger and Mihtash 1978.11
PR3 and MPO ANCA were done by ELISA kit of Euro diagnostica, Sweden supplied by MIS OSB agencies, Geeta Colony. For PR3, ELISA value more than 9.6 U/mL was considered as positive. For MPO ANCA, value above 12.7 U/mL was taken as positive. For anti GBM, value above 18 U/mL; for ANA, value above 1.2; for anti ds DNA, value above 46 IU/mL.
In CGN, blood vessels were destroyed & not visible due to severe mononuclear cell infiltrate. Hence, CD34 staining was done by immuneperoxidase method to see the endothelial cells of the blood vessels. Monoclonal Ab against CD34 and secondary antibody including substrate kit was purchased from Biogenex Emergo Europe, Molenstraat 15, NL-2513BH, Hague, Netherland supplied by MIS Biogenes.
Result
CGN included total of 50 cases. Sex wise distribution showed that males were slightly more affected (26 cases - 52%) than females (24 cases - 48%); although statistically it was non-significant.
Distribution of CGN revealed that 52% patients were in the 3rd and 4th decades of their life, 14% patients were children below 16 years of age and 34% were above 40 years. Age varied from 13 – 86 years. Age wise difference was significant (x2 = 21.52, P<.001). Mean age: 38.4 ± 16.9 years.
Subtypes of CGN revealed that Type III (PICGN) was more commonly found (60% cases) followed by Type IV CGN and Type V (12% each), Type I and Type II formed 8% each so as a whole 72% of CGN were ANCA positive and 20% were anti GBM positive.
All 36 ANCA positive CGN cases were further classified. It was found that maximum cases were of crescentic type (47.2%) followed by sclerotic type (25%), focal (16.7%) and mixed (11.1%).
All 50 CGN cases were further examined and subjected to various tests to know secondary causes. Evidence of SLE was found in 14 cases (28%). Out of these 14 cases, 64.28% were females and 35.71% males. Anti ds DNA Ab was positive in only 57.14% and anti Sm Ab was seen in 5 cases (35.71%). All patients fulfilled the criteria of SLE. MPO ANCA was positive in 50%, PR3 in 28.57% & anti GBM + anti MPO in 7.14% in SLE.
Out of 50 CGN cases, only 36 cases (72%) were ANCA positive. Out of these MPO ANCA was more common in both type III and IV CGN forming 53.33% and 66.66%, whereas PR3 ANCA was less common in both varieties (40% type III & 33.33% type IV). Two of these cases (6.6%) were dual ANCA positive for PR3 and MPO.
Most common histopathological findings in glomerulus were fibrocellular and fibrous crescents (Figure 1) found in 80% followed by hyalinization and sclerosis. In 34% cases, there were also cellular crescents. Bowman’s capsule necrosis was seen in 66.7% cases of Type IV and 60% cases of Type III and 33.3% cases of Type V CGN.
Granuloma in glomeruli were seen mostly in Type IV CGN and 25% cases of Type III and Type II CGN (Figure 2). RBC casts were seen mostly in Type IV CGN followed by Type V and Type I (80% each). Interstitium showed moderate to heavy mononuclear cell (MNC) infiltration in more than 75% of all types of CGN. Lymphoid follicles infiltration in interstitium is seen mostly in Type IV (33.3%) and Type I (25%) & Type V (16.7%). Non caseating epithelial granuloma was found in 25% cases of Type III, I and II and 16.7% cases of Type IV and V (Table 1).
In CGN, small blood vessels vasculitis was more common (60%) followed by fibromuscular hyperplasia (56%), hyaline thickening (44%), medium sized vessel vasculitis (22%), thrombosis (22%) and fibrinoid necrosis of blood vessels (16%). Small vessel vasculitis included glomerular and peritubular capillaritis. All peritubular vasculitis was not obvious because of inflammatory cells, only after CD34 staining destroyed capillaries were identified. In SLE, venulitis was more common. In Type I CGN, common findings were hyaline thickening (75%), fibromuscular hyperplasia (50%), small vessel vasculitis (50%), thrombosis in blood vessels, fibrinoid necrosis and medium sized blood vessel vasculitis (25%). Among Type II CGN only one case had vasculitis (25%) of small blood vessel. (Table 2) (Figure 3, Figure 4, Figure 5)
In Type III CGN small vessel vasculitis was more common (63.33%) whereas medium sized blood vessel vasculitis was seen in 20% cases and thrombus was found in 26.7% cases. As a whole vasculitis was seen in only 83.3%. In 2 cases (6.66%) epithelioid granuloma was found in blood vessel also. In Type IV, all cases showed vasculitis in which 50% showed small vessel and 50% showed medium sized blood vessel vasculitis. In type V small vessel vasculitis was present in 83.33%. One case of eosinophilic granulomatous polyangitis was a 40 years old female who manifested with acute renal failure, allergic rhinitis, proteinuria, haematuria, anaemia, leucocytosis and eosinophilia of 28%. Her MPO ANCA was positive and blood urea and serum creatinine were raised. Histopathology of kidney showed cellular crescents in more than 80% glomeruli, tubular necrosis, mononuclear cell infiltrate with many neutrophils and eosinophils in the interstitium. There was small vessel vasculitis with plenty of eosinophils.
Out of 41 cases, 8 cases (19.5%) of GPA were identified. Age varied from 17 years to 60 years (52 M, 33 M, 17 F, 55 M, 48 M, 32 F, 60 M, 36 M). Out of 8 cases, 6 (75%) were males and 2 (25%) were females.
Fever was present in 50% cases, rash and arthritis in 25% (2 cases). One case (12.5%) had otitis media with abscess in middle ear. Pulmonary symptoms were found in only 50% of the cases which comprised of breathlessness, cough, hemoptysis, nasal bleeding. All patients had swelling of face and feet, raised blood urea and serum creatinine. (Table 3)
All GPA patients had small vessel vasculitis (SVV) and granuloma (interstitium: 4 cases – 50%; glomeruli: 2 cases – 25%; blood vessel: 2 cases – 25%). Before CD34 staining, SVV was seen in 3 cases, but after staining, it was found in all cases.
Figure 1
Shows focal cellular crescent at 6 O’clock position whereas in other part it is fibrocellular. (H&E X 400)
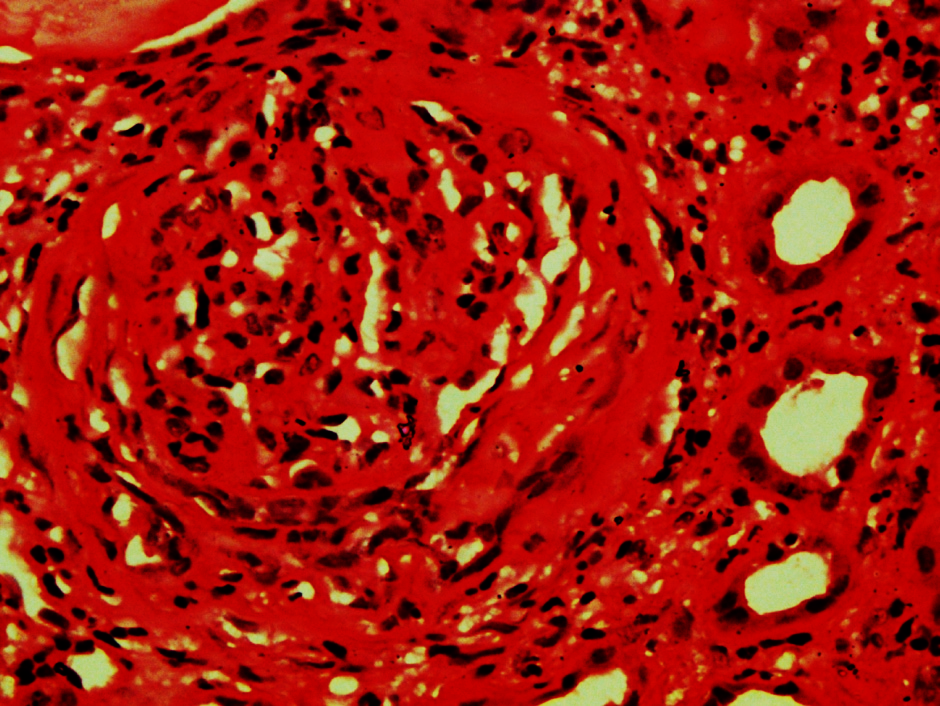
Figure 2
Showing small non caseating granuloma in periglomerular area of wegener’s granulomatosis. (H&E X400)
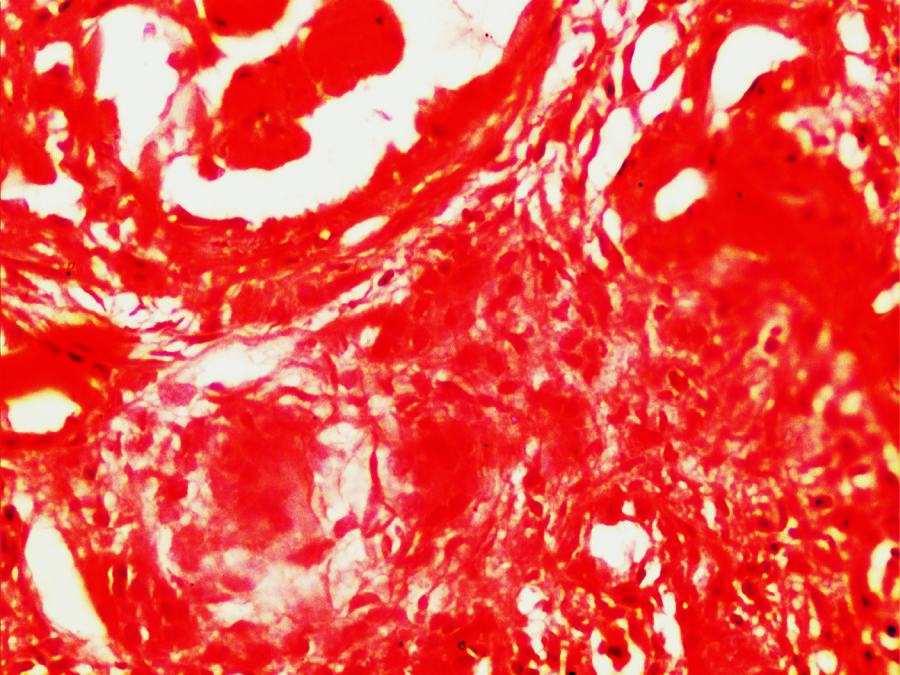
Figure 3
Showing severe vasculitis of peritubular capillaries in MPA with mononuclear cell infiltration on the left side but medium sized blood vessel on right side does not show vasculitis (H&E X 400)
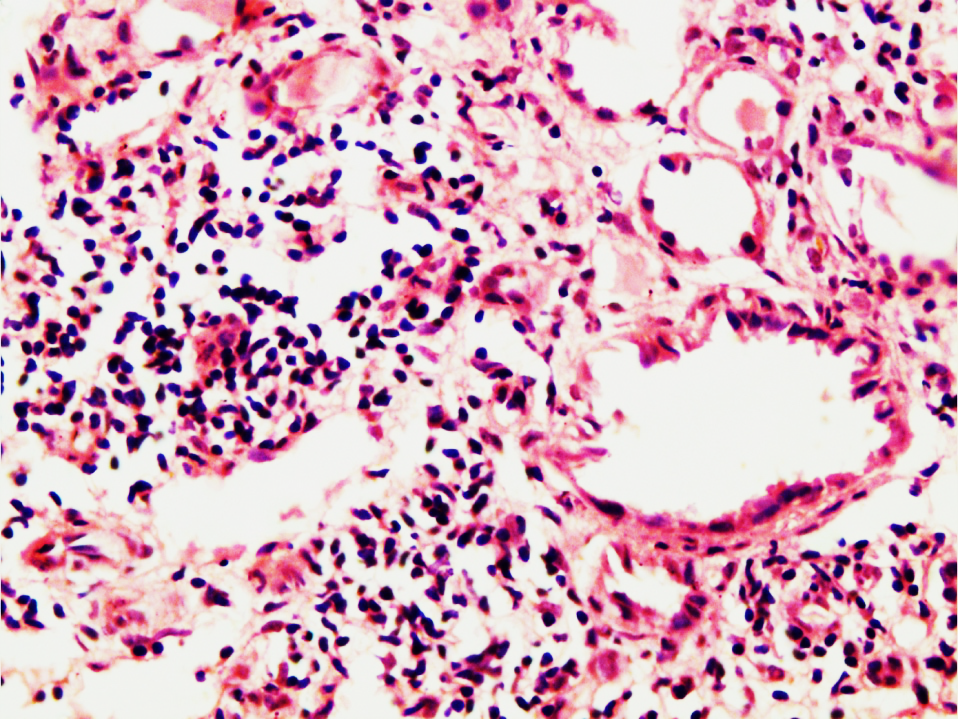
Figure 4
CD34 staining of venulitis showing destruction of endothelial cells (loss of CD34 staining) & heavy MNC infiltration. (IP staining with CD34 X400)
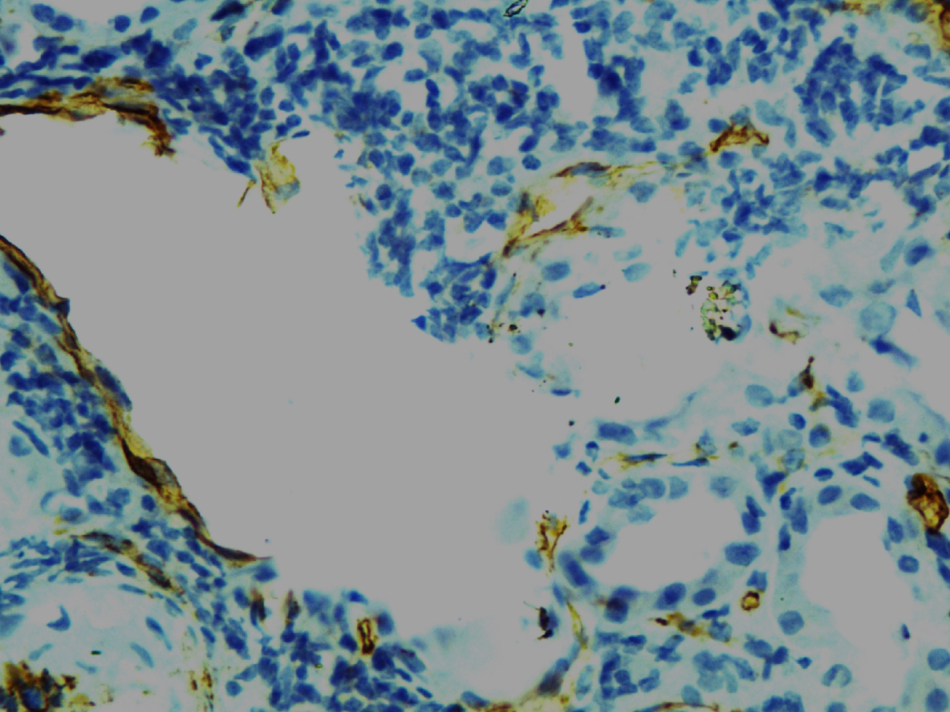
Figure 5
Showing fibrinoid necrosis of vessel wall (red colour), vasculitis & hyaline thrombi in lumina. (AFOG X 400)
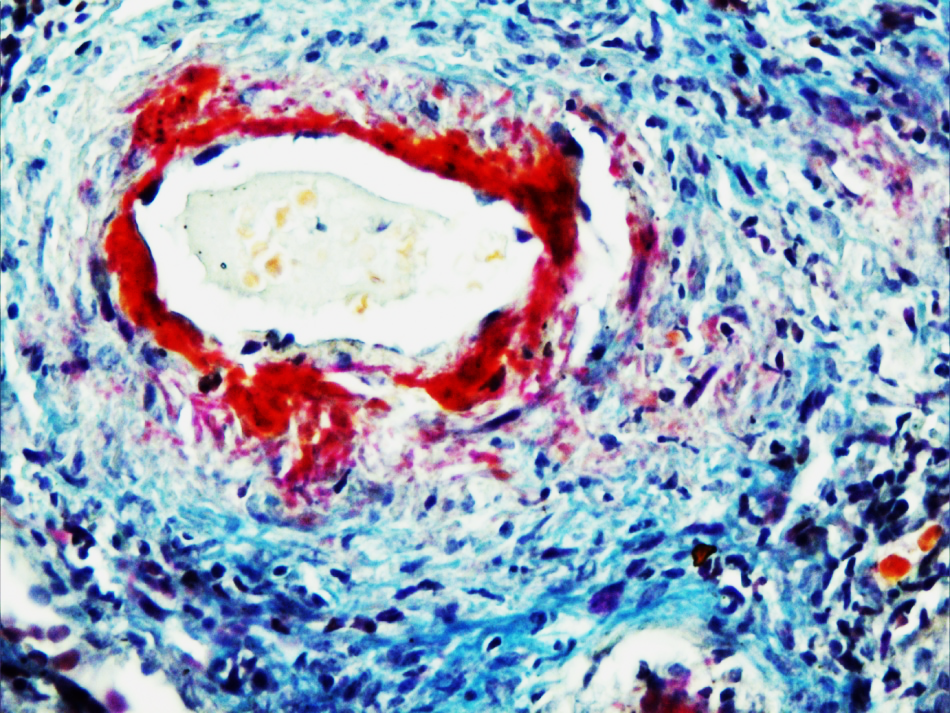
Table 1
Showing glomerular, tubular and interstitial pathology into various types of CGN
Table 2
Ishowing histopathological findings of blood vessels in various types of CGN
Table 3
Showing clinical features of GPA
Discussion
ANCA is important cause of vasculitis. Presence of ANCA is also reported in infections and malignancy such as myelodysplastic syndrome, Hodgkin lymphoma, SLE and systemic sclerosis.12 This is also an important cause of pauci-immune CGN. CGN is classified into 5 Types. Among them, one of the common type is Type III which is characterized by the absence of immune complexes hence known as Pauci-immune CGN. Majority of Type III CGN are ANCA positive.1 We studied 50 cases of CGN. Most common CGN was Type III which formed 60% of the cases while Type IV and Type V were seen in 12% cases each followed by Type I and Type II which formed 8% cases each. As a whole in more than 72% of CGN, ANCA was positive. There are variable reports regarding frequency of CGN, Gupta et al 201113 found ANCA negative pauci-immune CGN in 39.1% followed by ANCA positive pauci-immune (32.6%) and Type II CGN (28.3%). Like us Choudhury et al 201414 also found predominance of type III CGN (32.4%) followed by type II (23.5%), type V (18%), type I (14.5%) and type IV (11.7%) CGN.
Some studies indicate that type V CGN is very rare and comprises less than 5%.15, 16 While in our study it was seen in 12% cases.
In CGN, we found slight male predominance (52%) over females (48%) with male to female ratio of 1.1:1. Contrary to our study other workers found slight female predominance. Gupta et al 13 found male to female ratio of 1:1.1 while Choudhury et al14 found it to be 1:1.8. In our study, age range was from 13 - 86 years while mean age was 38.4 ± 16.9 years. More or less similar to our study mean age was 32.2 ± 16.1 years in series of Choudhury et al 2014.14 Age range in their series was 12 - 72 years and maximum cases (76.5%) were between 21 - 59 years, 11.7% cases were below 20 years and 11.7% cases were above 60 years. In our series 14% patients were below 16 years and 10% patients were above 61 years.
Contrary to this, Gupta et al 201113 found that in CGN age ranges from 4.5 to 72 years with mean age of 26.6 ± 17.1 years. In their series, 26.08% patients were below 14 years of age.
In present study, MPO positivity was seen in 50% CGN followed by PR3 positivity (44.4%). In 5.6% cases, both MPO and PR3 were positive. In series of Choudhury 14 et al, MPO ANCA was more common (81.8%) followed by PR3 ANCA and combined MPO and PR3 ANCA (9.1%) each.
We classified all 36 ANCA positive cases and found crescentic to be more common (47.2%). Similar to us, Naidu et al 201417 also reported crescentic to be more common while some studies (Ellis et al 201318 and Togashi et al 201619 found mixed variety to be more common.
Out of 50 CGN cases, vasculitis was seen in 41 (82%) cases. Commonest vasculitis was MPA (78.04%) followed by GPA in 19.5% and EGPA (2.44%). ANCA vasculitis with lupus nephritis was seen in 31.7% cases, immune complex vasculitis in 9.8% and ANCA negative pauci-immune vasculitis in 2.4% cases.
Naidu et al 201317 also reported higher incidence of GPA (56.6%) followed by MPA (40.8%). Vasculitis of interlobular arteries was seen in 50% of the cases of Type IV, 20% cases of Type III and 25% of the cases of Type II and 16.7% cases of Type V. Vizjak et al 20036 found active vasculitis in 23% of both MPO and PR3 ANCA positive cases. This difference may be due to the fact that they have noticed only medium sized blood vessel vasculitis. In our case also medium sized blood vessel vasculitis was present in 22% cases while peritubular capillaritis was missed in H&E stain but was visualized after CD34 staining.
One case was diagnosed as EGPA in a 40 years old female. This case was MPO positive. Similar to our case, Karamoto et al 201620 also reported a case of EGPA in a 64 years old female patient who had allergic rhinitis with sinusitis, breathlessness, pleural effusion, leucocytosis, eosinophilia, proteinuria, haematuria, raised blood urea and serum creatinine. Her case was also MPO positive and her renal biopsy revealed granuloma in the interstitium with MNC infiltration and eosinophilia.
Sinico et al 200621 reported that renal involvement is uncommon and is seen only 25% cases of EGPA. In CGN, we found evidence of GPA in 8 cases with mean age of 41 years with male predominance. Seven cases (87.5%) were PR3 positive and only one case (12.5%) was MPO positive. Pulmonary manifestations were seen in only 4 cases while nasal bleed was seen only in one case. Granuloma was present in all cases in which 50% cases were found in interstitium and 25% cases in glomeruli and rest 25% cases had it in blood vessels. Similar to our study, earlier work done in 267 cases of GPA with renal involvement22 also found mean age of 46 years with male predominance but in their study lung involvement was seen in 60.3% cases.
Aasarod et al 200122 studied 94 cases of GPA and found median age of 54 years with male predominance. About 75.5% cases were PR3 positive and 7.4% cases were MPO positive which is very close to our observation.
