- Visibility 182 Views
- Downloads 24 Downloads
- Permissions
- DOI 10.18231/j.ijpo.2020.030
-
CrossMark
- Citation
Clinicopathological study of spectrum of plasma cell dyscrasias in a tertiary care centre-retrospective four year study
- Author Details:
-
Sindhu Sharma
-
Jyotsna Suri *
-
Bhavneet Kour
Abstract
Plasma Cell Dyscrasias (PCD) by definition are represented by excessive proliferation of a single clone of cells producing entire immunoglobulins, immunoglobulin fragments, heavy chains or light chains. These encompass a wide range of disorders. In this study we try to discuss the clinical and pathological characteristics of this rare but important group of hematological malignancies.
Materials and Methods: This study was carried out in a tertiary care centre retrospectively for a period of four years. All the cases diagnosed with Plasma Cell Dyscrasias were selected. They were re-evaluated taking into consideration clinical aspects, radiological findings and blood and bone marrow examinations. These were further categorized using Salmon Durie Staging system.
Results: During this study a total of 41 cases fulfilled the diagnostic criteria of Plasma Cell Dyscrasias. Among them 33 were newly diagnosed cases of Multiple Myeloma, 4 cases were of relapse and 4 were of Waldenstrom’s Macroglobulinemia’s. Males outnumbered females in our study. The most common complaint was anemia (seen in 90% of cases) followed by lytic bone lesions and presence of M Band on electrophoresis. Deranged Renal Function Test were seen in 45.94 % of cases and hypercalcemia 54 % cases. Maximum cases were of stage III Multiple Myeloma (14) and 9 among them showed renal involvement as well.
Conclusion: Plasma Cell Dyscrasias are rare group of disorders, the diagnosis of which requires a systematic approach. The clinicopathological characteristic of different types of Plasma Cell Dyscrasias has varied presentations but Salmon Durie classification if applied carefully can help us to identify each type, evaluate and manage patients in a better way.
Introduction
Plasma cell dyscrasias (PCD) include a wide spectrum of disorders characterized by malignant proliferation of monoclonal population of plasma cells; which may or may not be accompanied by secretion of detectable levels of monoclonal immunoglobulins or paraproteins commonly referred to as M protein. This broad spectrum of Plasma Cell Dyscrasias include Monoclonal gammopathy of undetermined significance (MGUS), a symptomatic/ symptomatic Multiple Myeloma; Solitary Plasmacytoma of Bone; Extramedullary Plasmacytoma ; Waldenstrom’s Macroglobulinaemia (WM); Primary Amyloidosis and Heavy chain Disease.[2], [1]
The diagnosis of plasma cell dyscrasias involve evaluation of clinical burden of plasma cell infiltration, analysis of radiographically detectable bone lesions, electrophoretic determination of the monoclonal immunoglobulin and assessment of plasma cells in the bone marrow or extramedullary tissue.[3], [1] Recent developments and technological advances in detection of plasma cells pathology include Magnetic Resonance Imaging of bone lesions, immunophenotype detection of aberrant surface antigens on plasma cells, molecular characterization of clonally rearranged immunoglobulin genes and an expanding spectrum of cytogenetic abnormalities enhanced by fluorescent in situ hybridization technology.[2] Still morphological analysis of the bone marrow aspirate and trephine biopsy remains all time gold standard for quantifying the volume of medullary plasma cell infiltration and assessing the degree of Plasma Cell Dyscrasia.[4]
Waldenstrom’s macroglobulinemia (WM) is a lymphoplasmacytic lymphoma (LPL) that is characterized by high levels of monoclonal immunoglobulin M (IgM) protein in the blood. WM is one of low-grade B-cell lymphomas. Peripheral blood smear clearly exhibited rouleaux formation in these cases. It is often difficult to diagnose WM morphologically because the lymphoplasmacytoid LPL cells in the bone marrow can resemble mature lymphocytes or plasma cells.[6], [5]
In our present study we retrieved the data of patients referred to Hematology section of Department of Pathology, GMC Jammu with clinical complaints and general physical examination findings suggesting the possibility of diagnosis of Plasma Cell Dyscrasia or the cases in which routine PBF examination of case of anemia ; indicated a possibility/ strong suspicion of patient having a differential diagnosis of Plasma Cell Dyscrasia. Though most patients are symptomatic with complaints of bone pains, anemia, generalized weakness, lytic lesions of bones (may present at times as pathological fractures); 10-15% remain asymptomatic in whom treatment may be delayed. Many a times patients present with complications like anemia, infections, renal failure leading to morbidity. The present study was undertaken to evaluate the clinicopathological profile of patient diagnosed as Plasma Cell Dyscrasias on Bone Marrow Aspiration or Trephine Biopsy.
Materials and Methods
The present study is a cross sectional retrospective study conducted over a period of four years in a tertiary care centre after Institutional Ethics Committee clearance.
The patients referred to Hematology section of Department of Pathology, GMC Jammu by the clinicians from various specialties of hospital (mainly medicine, surgery, orthopedics) with a clinical suspicion /provisional / differential diagnosis of Plasma Cell Dyscrasia were included in the study. Also, cases diagnosed as Plasma cell Dyscrasia on BMA/Tr Bx were included in the study. The patients with failed aspiration or with inadequate material on BMA/ Trephine Bx (even after repeat procedure) were not included in our study.
The relevant clinical data regarding the chief complaints and radiological investigations including skeletal survey and imaging studies were obtained from records of hematology section and slides of cases included in our study were retrieved and Diagnosis reapproached based on Salmon Durie Criteria.
Patients common complaints of presentation were bone pains; lytic bone lesions; anemia low back ache; pathological fractures; vertebral compression. The values of Hematological investigations such as haemoglobin, total and differential cell counts, erythrocyte sedimentation rate, platelet count, peripheral blood smear, bone marrow aspiration / trephine biopsy were recorded. Ancillary test reports like Serum protein electrophoresis to detect M band, Urine for Bence Jones Proteinuria, FNA reports wherever applicable and available also provided supportive information. The BMA and Trephine biopsy slides of the cases under study were obtained from hematology section and restained wherever required. The diagnosis of all the cases was reapproached based on Salmon Durie Criteria. Complete data was compiled, categorized and statistics applied.
Results
Out of total 41 patients of Plasma cell Dyscrasia, 11 were females and 30 were male patients. One of the patient was 85 & other 93 years old. Maximum number of patients in our study were in sixth decade for both males & females. Mean age of patients with Plasma cell Dyscrasias was 64.65 years in our study ([Table 1]). Most common complaints of presentation in patients in our study were bone pains; lytic bone lesions; anemia; low back ache; pathological fractures; vertebral compression; fever; generalised weakness & fatigue. General physical examination of these patients revealed pallor and bone tenderness as common findings. The duration of symptoms in our study were on an average 5.8 months. Out of all 41 cases, diagnosed as either of Plasma Cell Dyscrasias ; 37 were cases of multiple myeloma; and 4 cases were diagnosed as Waldenstrom’s Macroglobulinaemia ([Table 2]).
Hematological investigations revealed Anemia in 90% of cases with Hb in range of 4-7 gm%. Erythrocyte sedimentation rate was raised in majority of patients to values as high as 140-160 mm/hr in 21 cases. On peripheral blood film examination, few common findings were b asophilic hue in PBF, rouleaux formation, pancytopenia / leucopenia / thrombocytopenia. Serum Urea and Creatinine were elevated to the values as high as 80-115 mg/d l and 10.6 -12.5 mg/dl respectively in majority of cases. Most of these patients had presented with complaints related to chronic renal parenchymal disease. Urinary Bence Jones Proteins were positive in 6 cases. Radiological examination revealed multiple osteolytic lesions in 25 cases; 5 patients had pathological fractures with most common sites of involvement being skull and spine.
28 patients had anemia according to the criteria satisfying myeloma related tissue or organ impairment (Hb <10 g/dl). Most of the cases (30) diagnosed as myeloma had total leucocyte count within normal range (n=30). 5 patients had leukocytosis and 2 presented with leucopenia. Five cases had thrombocytopenia. 30 patients showed increased ESR values ; 26 patients had values more than 100 i.e upto the range of 150-160 mm/hr. All patients diagnosed as MM had bone marrow plasmacytosis (> 10%) on marrow examination. 4 cases showed plasmacytosis in the range of 50-70%; 26 cases in the range of 30-50% and 7 cases in the range of 10-30-%.
Hypercalcaemia as we know is considered as one among important diagnostic criteria (CRAB Symptoms) was seen in 20 patients of MM. (Normal serum calcium level range being 8.5-10.2 mg/dl). 6 patients diagnosed as MM had hypocalcaemia; which could be due to Renal impairment or because of Vitamin D deficiency. Renal impairment was also seen in 16 patients evident by increased serum Creatinine levels (Normal range being 0.6-1.2 mg/dl). Serum IgG levels were increased in all 37 cases of MM in our study (Normal range being 0.62-1.4 g/dl).36 patients had a thick M band in serum protein electrophoresis and the most common site was the gamma globulin region ([Table 3]).
Applying Salmon Durie classification of staging of Multiple Myeloma; we found that there were 10 cases of stage 1 MM with 3 cases having renal involvement; 13 cases of stage 2 MM out of which only 4 cases showed renal involvement. Similarly, among 14 cases of stage 3 MM, 9 cases had serum creatinine values >2 mg/dl ([Table 4]).
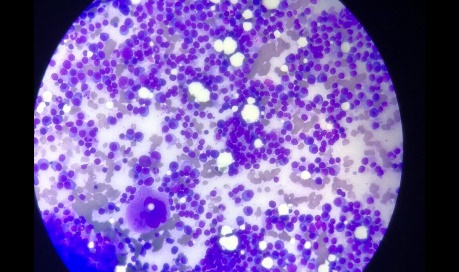
4 patients were of Waldenstrom’s Macroglobulinemia ([Figure 2]). These were the cases which had been referred with provisional clinical diagnosis of Plasma Cell Dyscrasia to hematology section but on laboratory investigations including PBF & Bone marrow aspiration were diagnosed as Waldenstrom’s Macroglobulinemia. All the four patients presented with complaints of fatigue, dizziness. On laboratory investigations all 4 patients were severely anemic with Hb values ranging from 4-5gm/dl; increased ESR upto 140 mm/ hr & rouleaux formation & plasmacytoid cells in PBF. Bone marrow aspiration showed moderately cellular smears comprising of >10 % lymphoplasmacytic cells infiltration and few mature plasma cells.

| Age group (in years) | Male | Female |
| 40-49 | - | 2 |
| 50-59 | 6 | 3 |
| 60-69 | 11 | 4 |
| 70-79 | 11 | 2 |
| 80-89 | 1 | - |
| 90-99 | 1 | - |
| Diagnosis | Male | Female | Total |
| Multiple myeloma | 28 | 9 | 37 |
| Waldenstroms macroglobulinaemia | 2 | 2 | 4 |
| Case | Hb %(g/dl) | S. Calcium Level (mg/dl) | Ig G Level (g/dl) | Skeletal Involvement | S. Creatinine Level (mg/dl) | Stage |
| 1 | 6 | 15 | 8.9 | Yes | 3.2 | III B |
| 2 | 6.5 | 7.4 | 5.56 | No | 3.78 | I B |
| 3 | 8.5 | 12 | 7 | Yes | 12.5 | III B |
| 4 | 8 | 5 | 2.4 | No | 1.1 | II A |
| 5 | 9.8 | 10.7 | 3.09 | Yes | 1 | II A |
| 6 | 4.2 | 12.5 | 9.4 | Yes | 2 | III B |
| 7 | 9.5 | 9 | 5.42 | No | 1 | I A |
| 8 | 6.2 | 11.9 | 8.4 | Yes | 0.2 | II A |
| 9 | 5 | 15.4 | 5.29 | Yes | 4.5 | III B |
| 10 | 9.5 | 9 | 3.5 | Yes | 1 | I A |
| 11 | 7 | 13.2 | 8.5 | No | 0.3 | III A |
| 12 | 12 | 6.5 | 4.2 | Yes | 0.9 | I A |
| 13 | 5 | 11.8 | 8.2 | Yes | 4.2 | III B |
| 14 | 4.3 | 12.5 | 13.4 | No | 1.09 | III A |
| 15 | 4.3 | 12.2 | 8.7 | Yes | 1.7 | III A |
| 16 | 5 | 13 | 10.4 | Yes | 4.9 | III B |
| 17 | 10.5 | 8.9 | 2.4 | Yes | 0.8 | I A |
| 18 | 13 | 9.6 | 2.78 | No | 1.04 | I A |
| 19 | 8 | 14.7 | 9.9 | No | 3.4 | III B |
| 20 | 9.6 | 8.8 | 6.8 | Yes | 0.7 | II A |
| 21 | 10.2 | 11.3 | 6.4 | Yes | 2.3 | II B |
| 22 | 3.8 | 10.4 | 7.7 | Yes | 1.1 | II A |
| 23 | 5.1 | 9.5 | 12.4 | No | 1.1 | II A |
| 24 | 7.9 | 13.6 | 8.84 | Yes | 2 | III B |
| 25 | 5.4 | 9.4 | 5.5 | Yes | 10.6 | II B |
| 26 | 6.5 | 8.7 | 8.5 | Yes | 1 | II A |
| 27 | 10.9 | 7 | 5.2 | Yes | 3.1 | I B |
| 28 | 4.3 | 14 | 13.4 | Yes | 2.3 | III B |
| 29 | 6.2 | 8 | 7.8 | No | 3 | II B |
| 30 | 6 | 10.1 | 5.42 | No | 1.2 | I A |
| 31 | 4.3 | 12.4 | 7.4 | No | 1.5 | III A |
| 32 | 14.4 | 9.5 | 2.5 | No | 1 | I A |
| 33 | 11 | 12 | 3.9 | Yes | 2.8 | I B |
| 34 | 10.5 | 8.2 | 12.2 | Yes | 4 | II B |
| 35 | 6 | 10 | 10.1 | No | 0.8 | II A |
| 36 | 4.8 | 15 | 12.1 | Yes | 1.5 | III A |
| 37 | 10 | 12.4 | 7 | Yes | 1.04 | II A |
| (a) One major criterion and one minor criterion or | (b) Three minor criteria in an individual who has signs or symptoms of multiple myeloma |
| Major criteria | Minor criteria |
| Plasmacytoma (as demonstrated on evaluation of biopsy specimen) | 10% to 30% plasma cells in a bone marrow sample |
| 30% plasma cells in a bone marrow sample | Minor elevations in the level of M protein in the blood or urine |
| Elevated levels of M protein in the blood or urine | Osteolytic lesions (as demonstrated on imaging studies) |
| Low levels of antibodies (not produced by the cancer cells) in the blood |
Discussion
Plasma cell Dyscrasias (PCD) are a heterogenous group of disorders in which there is expansion of clonal plasma cells which may produce monoclonal immunoglobulins. PCD’s include Monoclonal gammopathy of undetermined significance (MGUS); Asymptomatic/ symptomatic Multiple Myeloma; Solitary Plasmacytoma of Bone; Extramedullary Plasmacytoma ; Waldenstrom’s Macroglobulinaemia (WM); Primary Amyloidosis and Heavy chain disease. The etiology is still unknown; however, it has been related to chronic antigenic stimulation due to infection or with the exposure to specific toxic substances or radiation.[8], [7]
In our study covering a retrospective period of four years; we came upon cases of Multiple Myeloma (37) and Waldenstroms Macroglobulinaemia (4) only. The diagnosis of MM was made based on International Myeloma working group criteria for classification of Monoclonal Gammopathies, MM and related disorders ([Table 4]).[11], [10], [9]
Multiple Myeloma is hematological malignancy usually presenting in elderly age group and is unusual in younger age group. This is characterized by bone marrow infiltration with clonal plasma cells, production of monoclonal immunoglobulin (paraprotein); with associated complaints related to end organ damage –lytic lesions in bones, renal impairment, hypercalcemia and anemia. In our study, majority of patients; both males and females were in the age group of fifth, sixth and seventh decade; with 2 female patients of 40 and 45 years age and 2 male patients of the age of 85 and 93 years. The age incidence is similar to other studies.[13], [12], [1] The disease was more common in males as compared to females. Male to female ratio in our study is 2.73:1. Ries et al observed similar findings in their study that Plasma Cells Myeloma, bone and extraosseous plasmacytoma are more common in men than in women and the average age for myelomas was 54 years and 52 years for Plasmacytomas.[14] The clinical presentation of Plasma Cell Myeloma varies and symptomatic diagnosis is delayed by several years because of gradual involvement of the bone marrow by clonal proliferation of Plasma cells. This is comparable to what has been reported in various national and international studies.[16], [15]
The most common presenting complaint was fatigue and weakness which was mainly because of anemia, seen in 28 cases. Such patients also gave history of recurrent infections. 28 patients had anemia according to the criteria satisfying myeloma related tissue or organ impairment (Hb <10 g/dl). Anemia was the most frequent alteration found in studies performed by Kyle in the Mayo Clinic with 1027 patients.[17] The presence of anemia in patients results first by the replacement of bone marrow by neoplastic plasma cells and / or because of renal damage resulting from the loss of erythropoietin.
Renal impairment was seen in 16 patients with serum creatinine levels increased beyond normal range. This was again comparable to other studies done on MM. Renal manifestations of Plasma Cell Dyscrasia are common, are evident in approximately 25% of patients at the time of presentation and occur in 50% of patients at some point of disease. Renal involvement includes manifestations related to pathologic immunoglobulin itself or to the toxic effect of the light chain synthesized in excess. In addition, secondary metabolic disturbances play an increasingly important role. Finally, Renal manifestations include cast nephropathy, monoclonal Ig deposition disease and amyloid light chain deposition.[18]
As we know hypercalcemia is one of the diagnostic criteria laid down for MM, in our study also 20 patients presented with hypercalcemia which may be due to either renal impairment or bone disease (Vitamin D deficiency).[19] Hypercalcemia should be suspected in patients with Myeloma who have nausea, fatigue, confusion, polyuria or constipation. It may also suggest high tumour burden. Bone disease is very common in patients with MM and is one of the main cause of morbidity. Its clinical manifestations are lytic bone lesions and/ or osteoporosis which might be associated with pain, fractures, spinal cord compression and hypercalcemia. The pathogenesis of Myeloma bone disease is due to imbalance between bone resorption and bone regeneration due to inhibition of osteoblast induced bone formation and increased activity of osteoclast. Furthermore, the interactions in the bone marrow microenvironment may contribute to both disease evolution and bone disease.
The peripheral blood smear in a case of MM may reveal a normocytic, normochromic anemia with rouleaux formation. Similar were the PBF findings in our study where basophilic hue, rouleaux formation and normocytic normochromic picture predominated. Bone Marrow examination usually reveals an increase number of plasma cells. In our study 30 cases out of 37 cases showed bone marrow plasmacytosis in the range of 30-70%. These cells are positive for CD38, CD 138, MUM1 and a single class of cytoplasmic immunoglobulin. It is also seen that majority of the myeloma cells expressed CD40 and CD56 and are negative for CD5, CD19 and surface immunoglobulin expression. Monoclonality is frequently demonstrated by immunoperoxidase staining with Kappa and Lambda antibodies. The pattern of involvement in Plasma Cell Myeloma is macro focal so plasma cell count may be normal if the aspirate misses the focal aggregate of myeloma cells. Therefore, guided marrow biopsies yield commensurably high plasmacytosis.[4]
Serum Electrophoresis in our study revealed increased IgG levels in 37 cases (90%) and increased IgM in 4 cases (9.7%) which is in contrast to the usual percentages observed in the literature wherein, IgG myeloma constitute 60% and IgM Myelomas are very rare. Waldenstrom’s Macroglobulinemia is a distinct entity with positive M band for IgM monoclonal protein and at least 10% lymphoplasmacytic lymphoma cells in the marrow. Marrow infiltration is by small lymphocytes, plasmacytoid cells and plasma cells, diffuse, interstitial or nodular pattern of bone infiltration and surface Ig+, CD5-, CD10-, CD19+, CD20+, CD23- immunophenotype. It is accepted that WM can be distinguished clinically from IgM myeloma by the lymphoplasmacytic versus pure plasmocytic morphology, absent versus present bone involvement, and immunophenotypic findings.[20], [6], [5]
Before the beginning of systemic therapy for MM, all the patients with symptomatic disease should be staged using either Samon Durie system at diagnosis or the International Staging System. The Durie Salmon Staging system better provides the information on tumour burden whereas ISS serves better as a prognostic indicator. Being associated with Pathology, we have done Salmon Durie Staging System to get a better hold at the diagnostic platform.[13], [12]
Source of funding
None.
Conflict of interest
None.
References
- Jacob LA, Babu MCS, Lakshmaiah KC, Babu KG, Lokanatha D, Rajeev LK. Multiple myeloma: Experience of an institute in limited resource setting. Indian J Cancer. 2017;54:340-342. [Google Scholar]
- Lee JH, Lee DS, Lee JJ, Chang YH, Jin JY, Jo DY. Multiple myeloma in Korea: Past, present, and future perspectives. Experience of the Korean Multiple Myeloma Working Party. Int J Hematol. 2010;92:52-57. [Google Scholar]
- Munshi NC, Longo DL, Anderson KC, Kasper DL, Fauci AS, Longo DL, et al. Plasma cell disorders. Harrison's Principles of Internal Medicine. 19th ed. 2015. [Google Scholar]
- Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374:324-339. [Google Scholar]
- . International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: A report of the International Myeloma Working Group. Br J Haematol. 2003;121:749-757. [Google Scholar]
- Vakil NB, Agarwal MB, Tilve GH. Waldenstrom's macroglobulinemia. (A case report). J Postgrad Med. 1982;28:238-238. [Google Scholar]
- Dudala SR, Reddy KA, Prabhu GR. Prasad's socio-economic status classification - An update for 2014. Int J Res Health Sci. 2014;2:875-878. [Google Scholar]
- . Cancer Statistics, Cancer Research UK. . 2013. [Google Scholar]
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:538-548. [Google Scholar]
- Greipp PR, Miguel JS, Durie BG, Crowley BG, Barlogie B, Bladé J. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412-3420. [Google Scholar]
- Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L. Revised international staging system for multiple myeloma: A report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863-2869. [Google Scholar]
- Fousad C, Gangadharan KV, Abdulla MC, Naryan R, MJMA. Clinical profile of multiple myeloma in South India. Indian J Med Paediatr Oncol. 2018;39:62-66. [Google Scholar]
- Bora K. Distribution of multiple myeloma in India: Heterogeneity in incidence across age, sex and geography. Cancer Epidemiology. 2019;59. [Google Scholar]
- Guadarrama MBR, Medina C, Martinaz EA. Plasma Cell Neoplasms, Clinicopathological Characterstics and Immunophenotyping of 21 patients. Open J Pathol. 2012;2:127-132. [Google Scholar]
- Kim K, Lee JH, Kim JS, Min CK, Yoon SS, Shimizu K. Clinical profiles of multiple myeloma in Asia-An Asian Myeloma Network study. Am J Hematol. 2014;89:751-756. [Google Scholar]
- Kaur P, Shah BS, Baja P. Multiple myeloma: A clinical and pathological profile. Gulf J Oncolog. 2014;1:14-20. [Google Scholar]
- Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21-33. [Google Scholar]
- Sridhar S, Dutta TK, Basu D. Clinical profile of multiple myeloma and effect of thalidomide based treatment on its outcome. J Indian Med Assoc. 2011;109:880-882. [Google Scholar]
- Ramasamy I. Hypocalcemia in multiple myeloma secondary to unrecognized Vitamin D deficiency: A case report. Bone. 2011;48:27-28. [Google Scholar]
- Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. . 2001. [Google Scholar]
How to Cite This Article
Vancouver
Sharma S, Suri J, Kour B. Clinicopathological study of spectrum of plasma cell dyscrasias in a tertiary care centre-retrospective four year study [Internet]. Indian J Pathol Oncol. 2020 [cited 2025 Oct 07];7(1):158-163. Available from: https://doi.org/10.18231/j.ijpo.2020.030
APA
Sharma, S., Suri, J., Kour, B. (2020). Clinicopathological study of spectrum of plasma cell dyscrasias in a tertiary care centre-retrospective four year study. Indian J Pathol Oncol, 7(1), 158-163. https://doi.org/10.18231/j.ijpo.2020.030
MLA
Sharma, Sindhu, Suri, Jyotsna, Kour, Bhavneet. "Clinicopathological study of spectrum of plasma cell dyscrasias in a tertiary care centre-retrospective four year study." Indian J Pathol Oncol, vol. 7, no. 1, 2020, pp. 158-163. https://doi.org/10.18231/j.ijpo.2020.030
Chicago
Sharma, S., Suri, J., Kour, B.. "Clinicopathological study of spectrum of plasma cell dyscrasias in a tertiary care centre-retrospective four year study." Indian J Pathol Oncol 7, no. 1 (2020): 158-163. https://doi.org/10.18231/j.ijpo.2020.030
